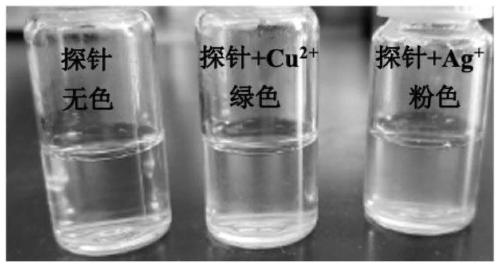
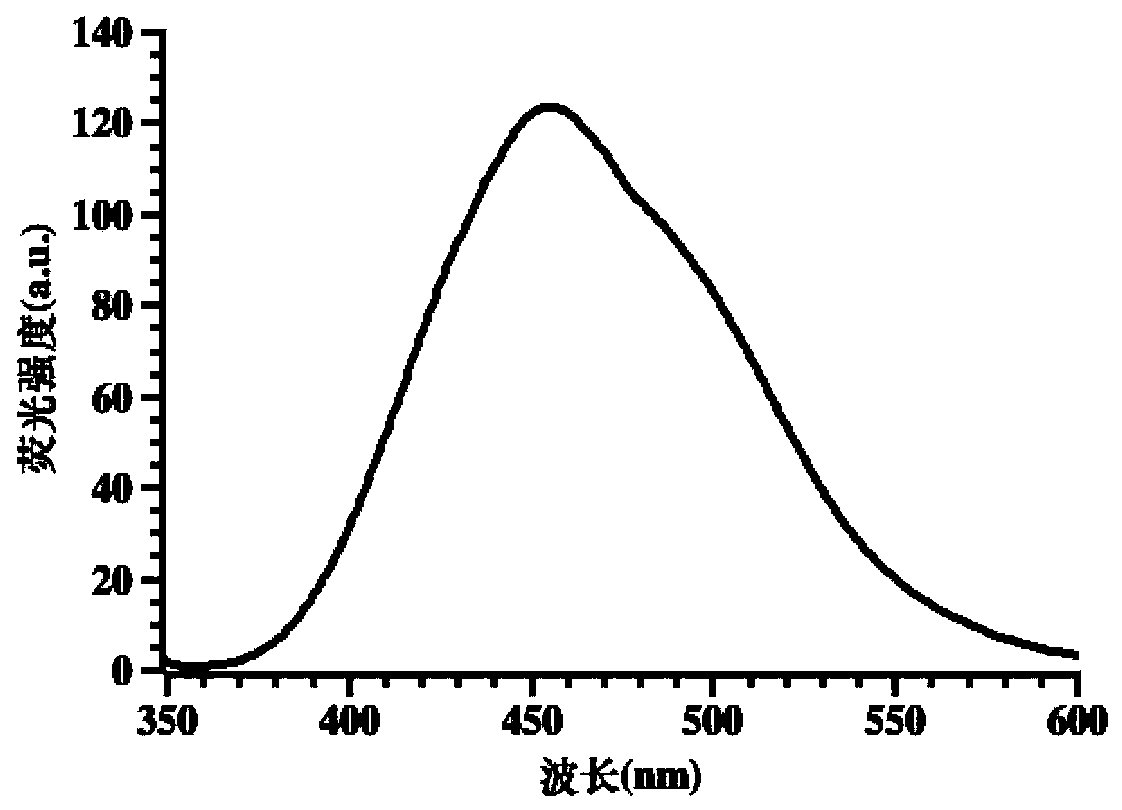
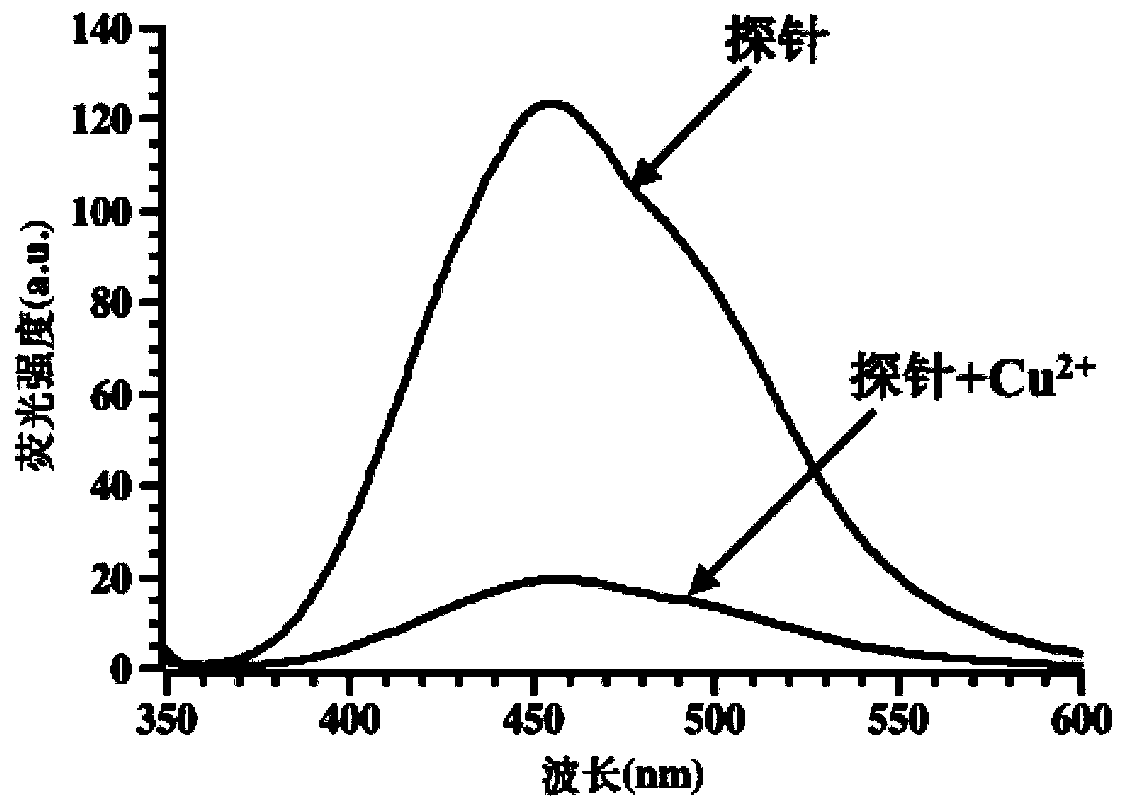
Double-colorimetric double-fluorescent phenanthroimidazole probe as well as preparation method and application thereof
A phenanthroimidazole and dual-fluorescence technology, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials, can solve the problems of inability to realize quantitative detection, single response object of colorimetric probe, and slow response speed, etc. Qualitative and quantitative detection, high sensitivity, rapid response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0040] Specific embodiment one: the double colorimetric double fluorescence phenanthroimidazole probe of this embodiment, its structural formula is:
[0041]
specific Embodiment approach 2
[0042] Specific embodiment two: the method for preparing the double colorimetric double fluorescent phenanthroimidazole probe described in specific embodiment one, carries out according to the following steps:
[0043] Add 2-(4-aminophenyl)phenanthroimidazole and m-phthalaldehyde into the organic solvent I according to the ratio of substance molar ratio (1~6):1, under the condition of temperature 20~80℃ React for 1 to 8 hours, during the reaction, solids are precipitated; after the reaction, filter, wash the filter cake with organic solvent II and distilled water successively, and dry the filter cake to obtain a crude product; recrystallize the crude product with organic solvent III to obtain double ratio Chromatic dual fluorescent phenanthroimidazole probe.
specific Embodiment approach 3
[0044] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the organic solvent I is glacial acetic acid, methanol, ethanol, N,N-dimethylformamide, benzene or toluene; others are the same as Embodiment 2 same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com