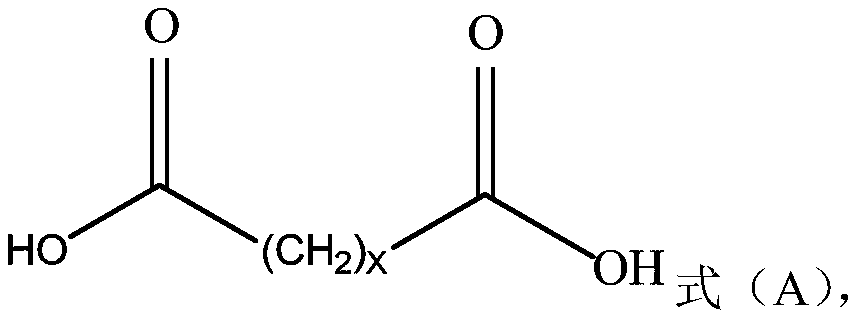
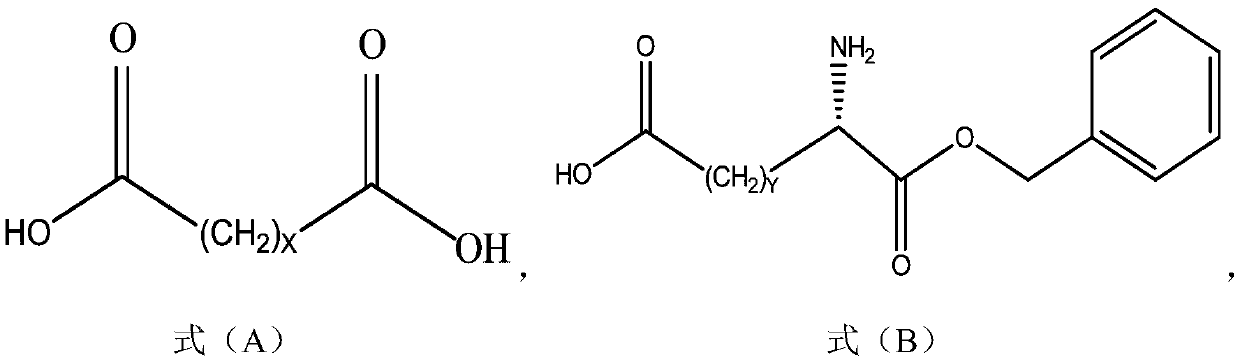
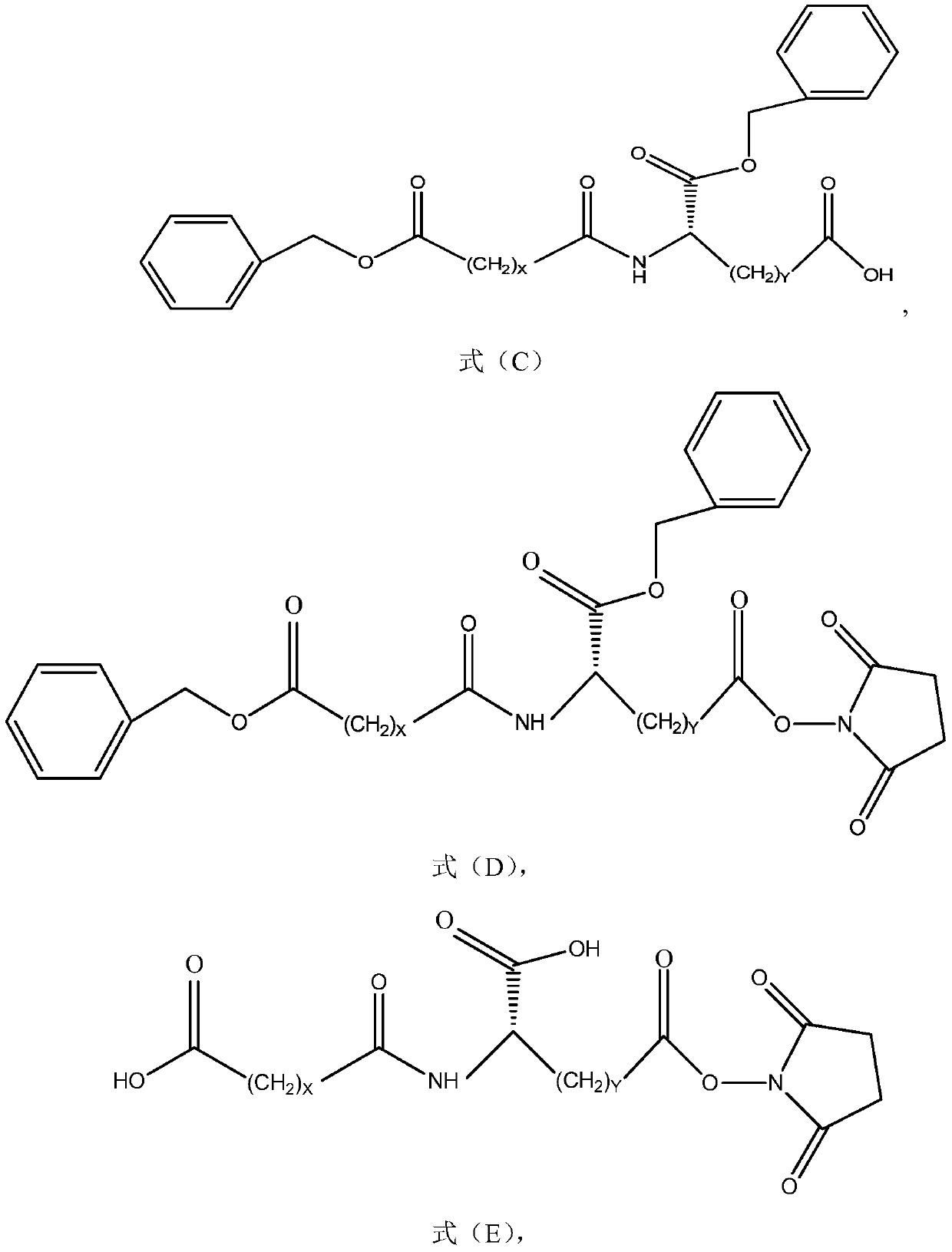
Method for preparing long-chain fatty diacid monobenzyl ester and application of long-chain fatty diacid monobenzyl ester
The technology of monobenzyl diacid and chain dibenzyl ester is applied in the field of biopharmaceuticals, and can solve the problems of difficulty in realizing industrial scale-up, high cost of chemical reagents, large preparation cost, etc., and achieves low post-processing cost, low preparation cost, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The method for preparing long-chain fatty diacid monobenzyl ester according to an embodiment of the present invention can be as follows:
[0055]
[0056] 1-1) Compound A, benzyl alcohol, and catalyst a are prepared in solvent b to obtain intermediate A'. After the reaction is completed, cool down to room temperature, then add reverse solvent c to the reaction system, and continue to cool down to about 0°C to crystallize. Filter and wash the filter cake with reverse solvent c to obtain compound B with a purity of more than 95%, which can be used in the next step after drying.
[0057] 1-2) Compound A' and (acid) base d are stirred and reacted in solvent e. After the reaction is completed, (acid free and solvent extraction can be skipped if the reaction is hydrolyzed with acid) after acid f is free, solvent g is extracted, and the Add the reverse solvent h to the organic phase to crystallize, filter, rinse the filter cake with the reverse solvent h, and dry the filter...
Embodiment 1
[0088] The synthesis of embodiment 1 hexadecanedioic acid dibenzyl ester
[0089]
[0090] Add hexadecanedioic acid (100.0g, 349.4mmol), benzyl alcohol (167.4g, 1547.8mmol), TsOH (6.0g, 34.9mmol) and 1000mL toluene into a 3000mL three-necked flask, place it at 120°C and stir for 24h, the reaction is over , cooled to room temperature, with 0.5M Na 2 CO 3 .aq (35ml, 17mmol) washes the reaction system, separates and discards the aqueous phase. Add 1000ml of n-heptane to the organic phase under stirring. With the addition of n-heptane in the system, solids are gradually precipitated in the system. The crystallization continued in the tank, and the temperature of the low-temperature tank was gradually lowered to -5°C. When the temperature of the system dropped to -5°C, the system was kept and stirred for 1 hour to allow the system to crystallize completely. After filtering, the filter cake was washed with 200 ml of n-heptane, and the filter cake was vacuum-dried at 40° C. for...
Embodiment 2
[0093] Synthesis of Monobenzyl Hexadecandioate (a)
[0094]
[0095]Add dibenzyl hexadecanedioate (4.7g, 10mmol) and benzyl alcohol (25ml) into a 100mL one-necked flask, stir and dissolve at room temperature, then add KOH (0.55g, 11mmol) dropwise to the reaction system under stirring at room temperature Benzyl alcohol (10ml) solution, after the dropwise addition, was stirred overnight at room temperature. Use 1M HCl.aq (25ml) to adjust the pH of the reaction system: 1-2, filter, separate and discard the aqueous phase. At room temperature, n-heptane (40ml) was added dropwise to the organic phase, and a white solid began to precipitate out in the system along with the addition of n-heptane. Transfer the reaction system to a low-temperature tank at 0°C, and continue to cool down to crystallize. After the system cools down to 0°C, more solids are precipitated in the reaction bottle, and continue to heat and stir for 0.5h. Filter at low temperature and rinse twice with n-hepta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com