miRNA marker related to liver cancer sorafenib resistance and application of miRNA marker
A sorafenib and fenib-resistant technology, applied in the fields of biology and oncology, can solve problems such as unclear mechanism, and achieve the effect of strong applicability, reliable source and easy detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
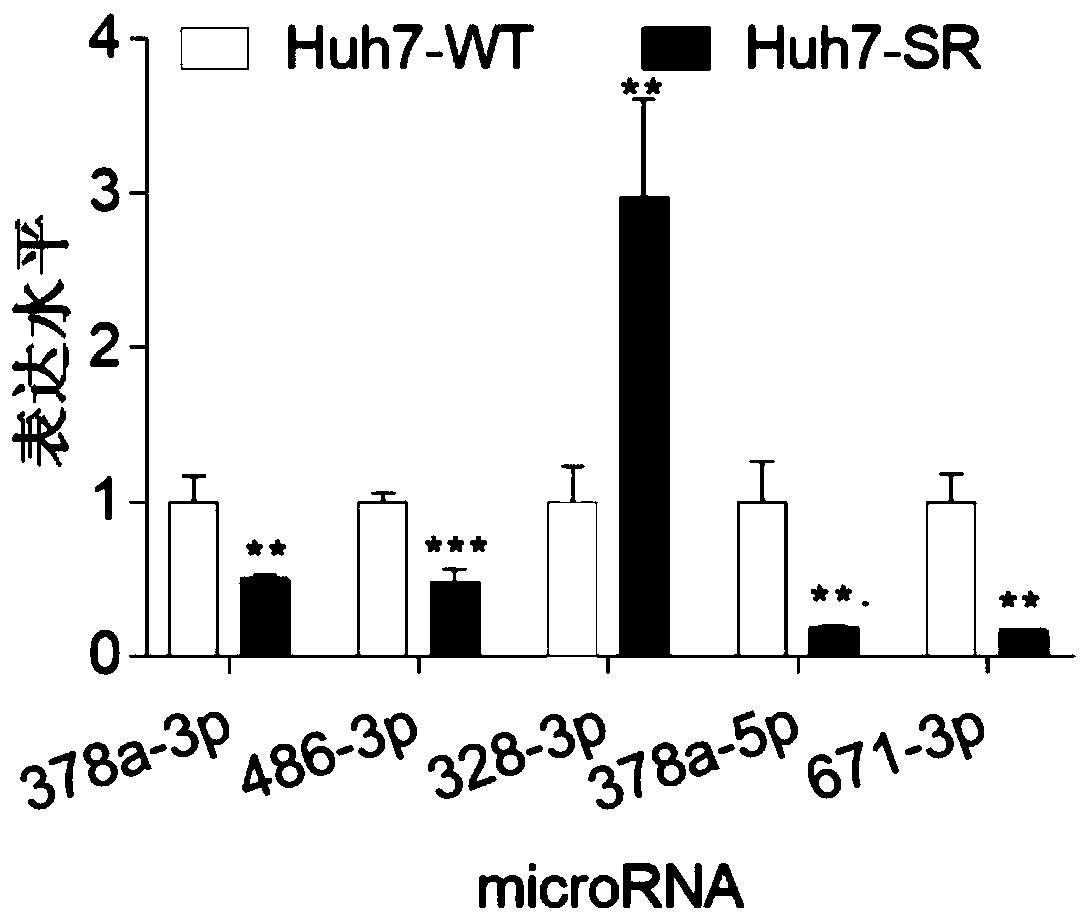
[0045] Example 1 miRNA screening of liver cancer sorafenib resistance
[0046] Total RNA Extraction
[0047] (1) Add Trizol to Sorafenib-resistant Huh7 cell line and normal Huh7 cell line, and store at room temperature for 5 minutes
[0048] (2) Add 0.2ml of chloroform, mix thoroughly after shaking, and place at room temperature for 5-10min
[0049] (3) Centrifuge at 12,000 rpm for 15 minutes at high speed, transfer the upper aqueous phase to another new centrifuge tube, and be careful not to absorb the protein between the two aqueous phases. Transfer to a new tube, add an equal volume of -20 ℃ pre-cooled ℃ pre-cooled isopropanol, fully invert and mix, and place on ice for 10 minutes;
[0050] (4) After centrifuging at 12000rpm for 15min at high speed, carefully discard the supernatant, add 75% DEPC ethanol at a ratio of 1ml / ml Trizol to wash the precipitate (store at 4°C), wash the precipitate, shake and mix, and centrifuge at 12000rpm at 4°C for 5min at high speed;
[005...
Embodiment 2
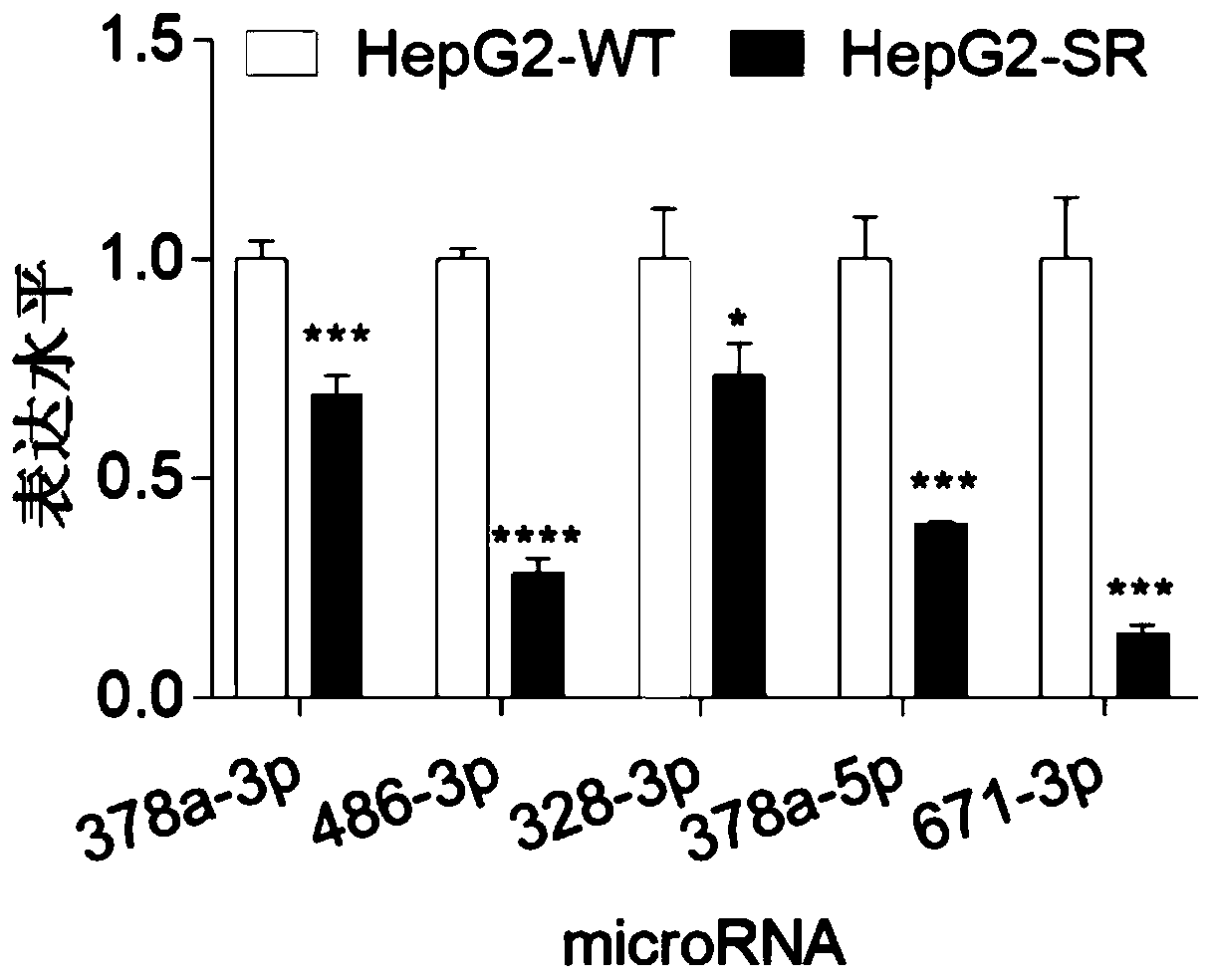
[0054] Example 2 QPCR verification of differentially expressed miRNAs
[0055] (1) According to the detection results of the miRNA chip, miRNA378a-3p, miRNA-486-3p, miRNA-328-3p, miRNA-378a-5p, and miRNA-671-3p were selected for QPCR verification on cell lines. According to the total RNA extraction method in Example 1, Sorafenib-resistant Huh7 and normal Huh7, Sorafenib-resistant HepG2 and normal HepG2,2 were subjected to total RNA extraction from liver cancer cell lines.
[0056] (2) reverse transcription RNA
[0057] Mix 10pg-1μg of total RNA template with 2μl 10* buffer, 2μl dATP (10mM), 0.5μl polyA polymerase, 0.5μl ribonuclease (RNase) inhibitor, and RNase free water (RNase free water), volume last 20 μl and incubated at 37°C for 1h. Then add 1 μl 0.5 μg / μl Oligo (dT)-specific RT primer (purchased from GeneCopoeia, Cat. No. QP017 / QP018) to the reaction tube, incubate at 70°C for 5 min and immediately incubate on ice for at least 2 min to interrupt the interaction betwee...
Embodiment 3
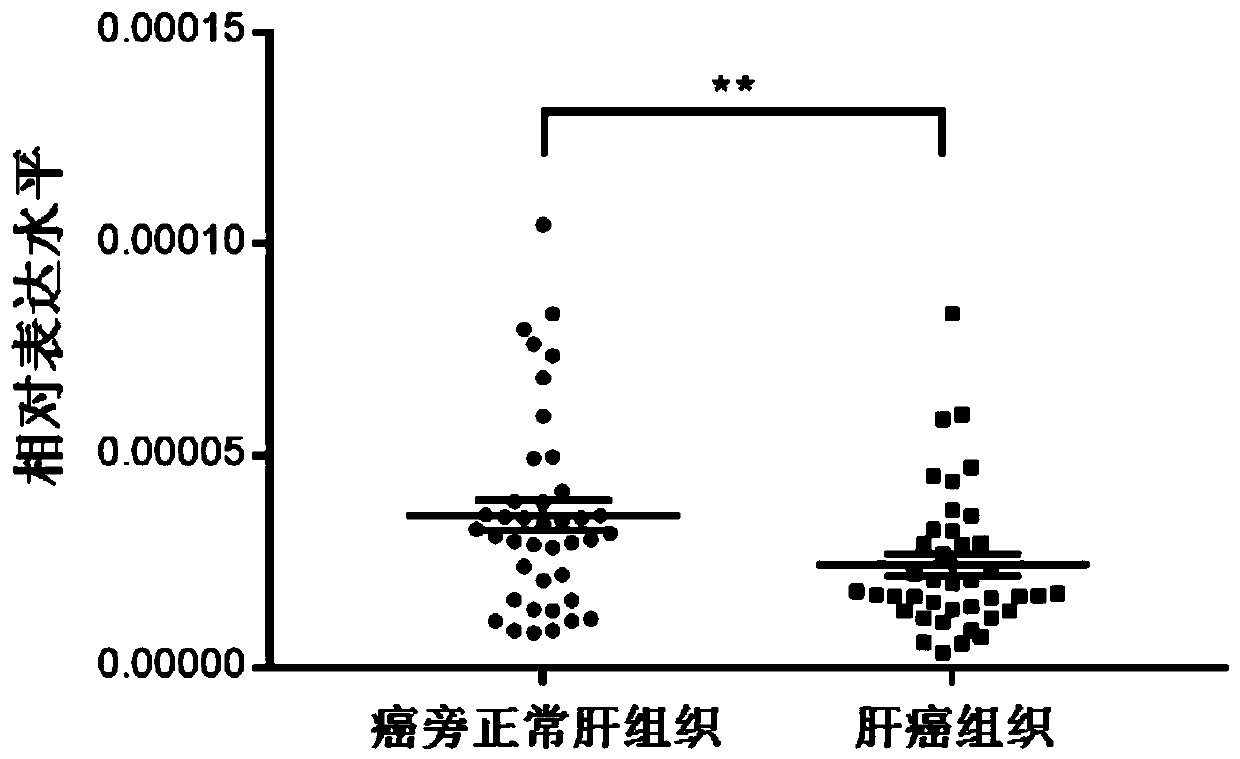
[0062] The inventor collected liver cancer tissue specimens and adjacent normal liver tissue from 40 liver cancer patients in Run Run Shaw Hospital affiliated to Zhejiang University School of Medicine. All 40 patients were treated with sorafenib. The expression level of miRNA-486-3p in liver cancer tissues and adjacent tissues was detected.
[0063] After liver cancer tissue and paracancerous tissue were homogenized and crushed, total RNA was extracted according to the total RNA extraction step in Example 2. Follow the above steps to perform reverse transcription of RNA into cDNA, QPCR reaction, and result analysis. The result is as image 3 As shown, the expression of miRNA-486-3p in liver cancer tissues treated with sorafenib was significantly lower than that in adjacent normal tissues.
[0064] The relationship between miRNA-486-3p and survival and prognosis of liver cancer was analyzed in the liver cancer database. Such as Figure 4 As shown in Table 1, it was found t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com