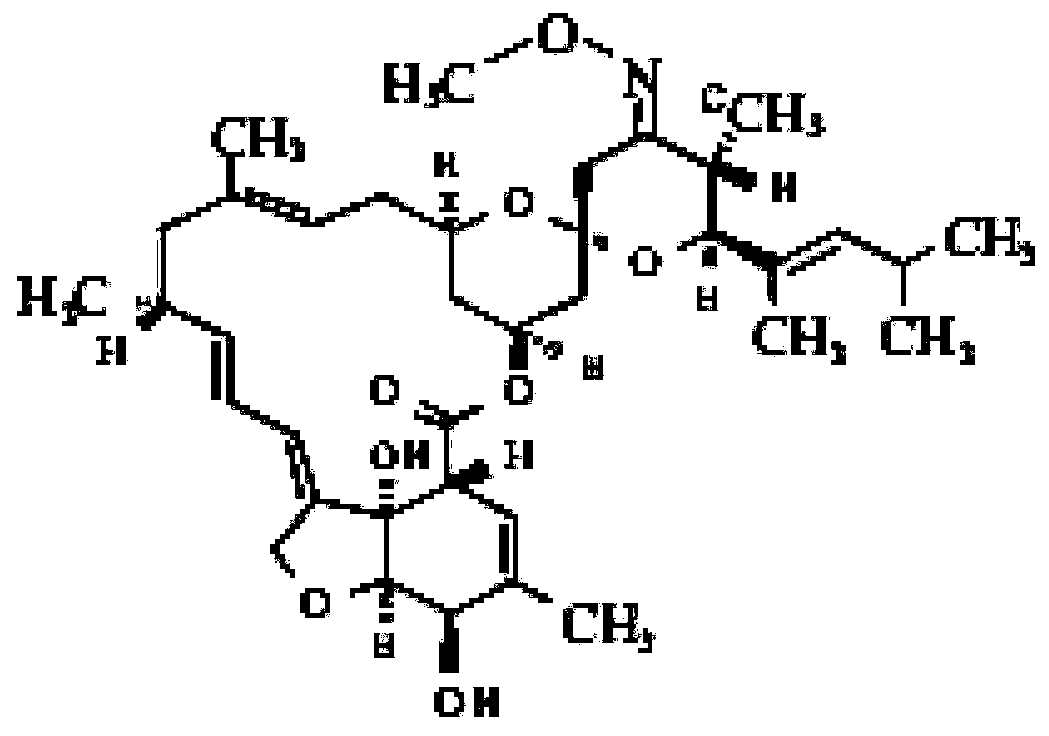
Slow-release gel preparation containing moxidectin for injection and preparation method of preparation
A technology of moxidectin and sustained-release gel is applied in the field of sustained-release gel preparation and preparation for injection containing moxidectin, which can solve problems such as difficulty in preventing and controlling the spread of parasites across regions, and achieve long-term maintenance of drug effect. , The preparation method is simple, and the biocompatibility is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
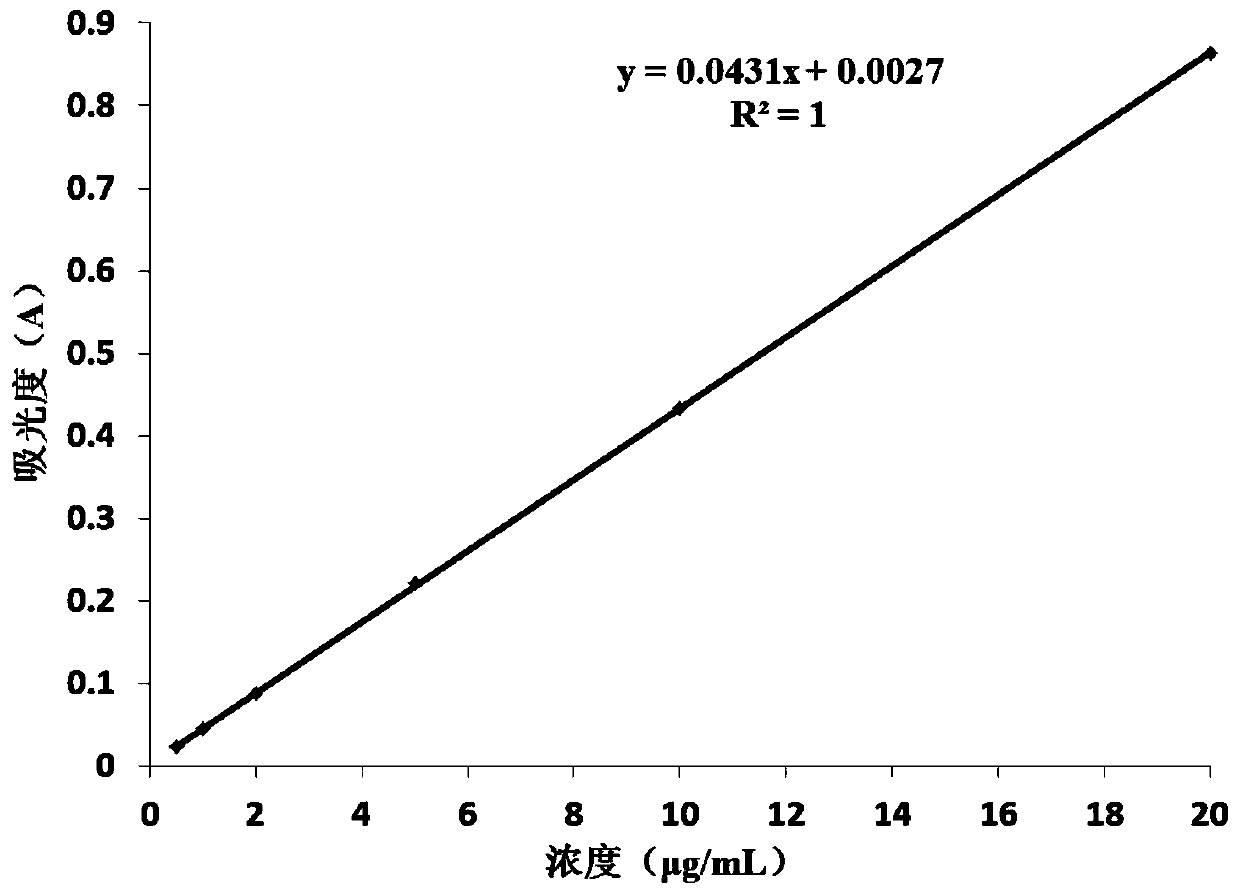
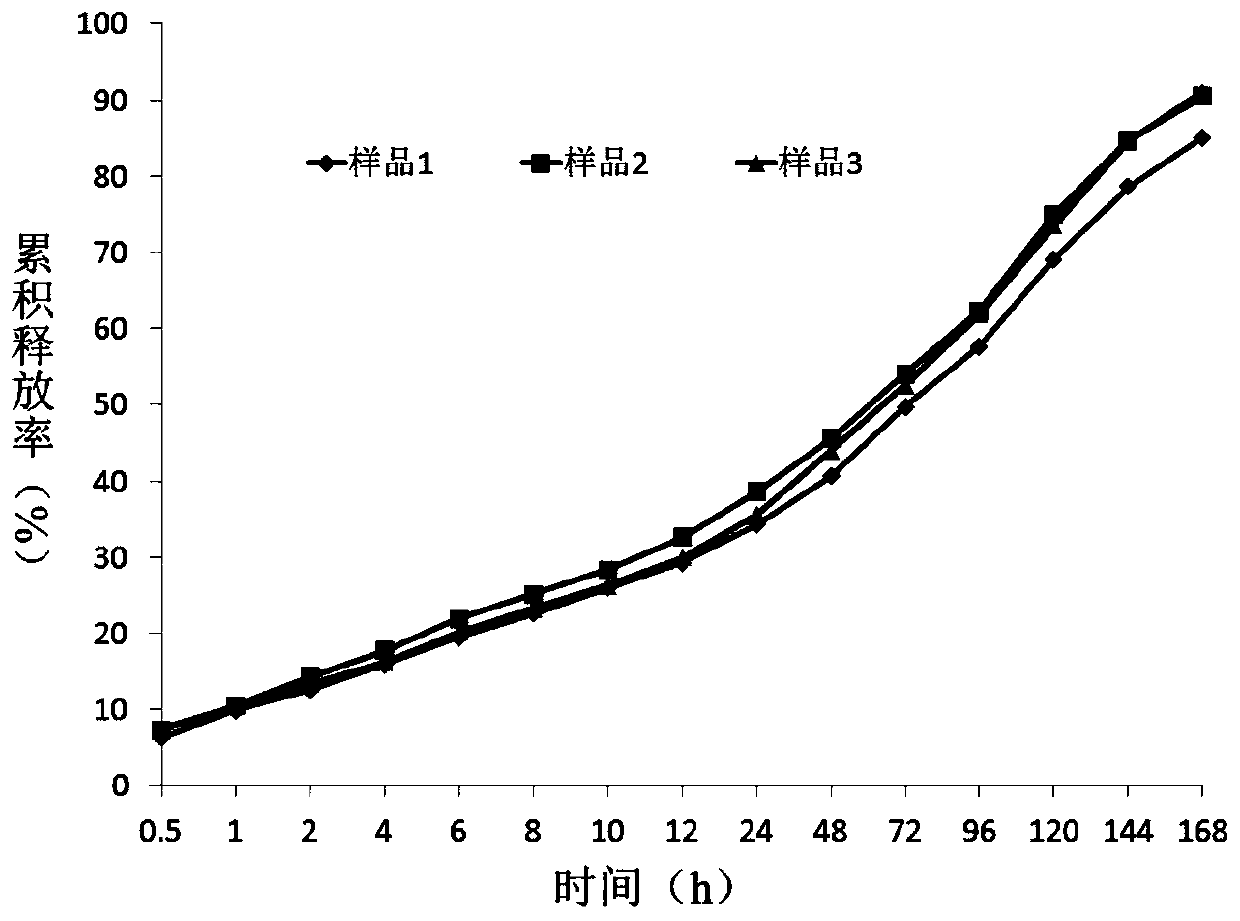
Image
Examples
Embodiment 1
[0028] Dissolve 0.2g of butylhydroxyanisole and 0.3g of 2,6-di-tert-butyl-p-cresol into 1mL of ethanol, add an appropriate amount of sterilized pure water, and stir to dissolve. Add 5 g of moisturizing agent sorbitol to the solution, after mixing, add 1 g of slow-release agent sodium alginate at room temperature, and stir to dissolve. Add 0.05 g of benzalkonium bromide preservative to the solution, and stir to dissolve. Add 0.5 g of sterile moxidectin to the solution after filter membrane sterilization, and disperse at a high speed of 20,000 rpm for 10 minutes under a sterile environment. Then, 18% of P407 and 1% of P188 were added after sterilized treatment, and left to swell naturally at 4°C for 48 hours. Finally, sterilized pure water was added to make the volume to 100 mL, and mixed evenly to obtain a 0.5% moxidectin sustained-release gel preparation for injection.
Embodiment 2
[0030]Dissolve 0.5g of 2-tert-butylphenol into 1mL of ethanol, add an appropriate amount of sterilized pure water, and stir to dissolve. Add 10 g of humectant PEG 400 to the solution, and after mixing, add 0.05 g of preservative benzyl alcohol to the solution, and stir to dissolve. After the solution is sterilized by a 0.45 μm filter membrane, the temperature of the solution is raised to 60°C, and 0.1 g of the slow-release agent polyethylene glycol-polylactic acid-polyethylene glycol triblock copolymer is added under sterile conditions, and after stirring and mixing , Add 1 g of sterile moxidectin to the solution, and disperse at a high speed of 20,000 rpm for 10 minutes. Then, return to room temperature, add 18% P407 and 1% P188 after sterilization, and let it swell naturally at 4°C for 48h. Finally, sterilized pure water was added to make the volume to 100 mL, and mixed evenly to obtain a 1% moxidectin sustained-release gel preparation for injection.
Embodiment 3
[0032] Dissolve 0.5g of 2,6-di-tert-butyl-p-cresol into 1mL of ethanol, add an appropriate amount of sterilized pure water, and stir to dissolve. Add 5 g of moisturizing agent sorbitol to the solution, after mixing, add 2 g of slow-release agent methylcellulose at room temperature, and stir to dissolve. Add 0.05 g of benzalkonium bromide preservative to the solution, and stir to dissolve. Add 2 g of sterile moxidectin to the solution after filter membrane sterilization, and disperse at a high speed of 20,000 rpm for 10 minutes under a sterile environment. Then, add 22% P407 and 1% P188 after sterilization, and let it swell naturally at 4°C for 48-72h. Finally, sterilized pure water was added to make the volume to 100 mL, and mixed evenly to obtain a 2% moxidectin sustained-release gel preparation for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com