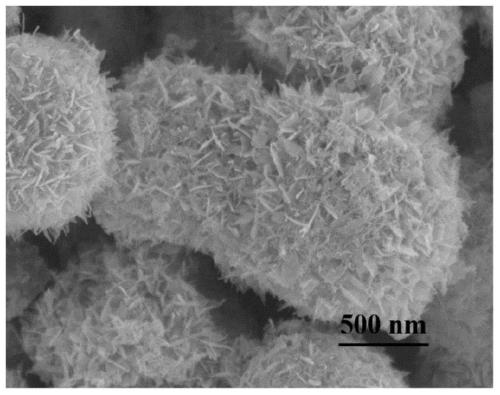
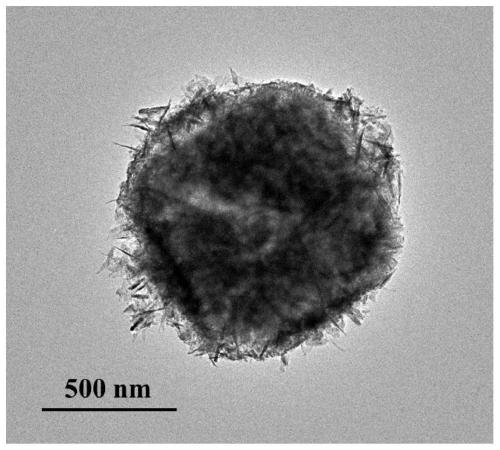
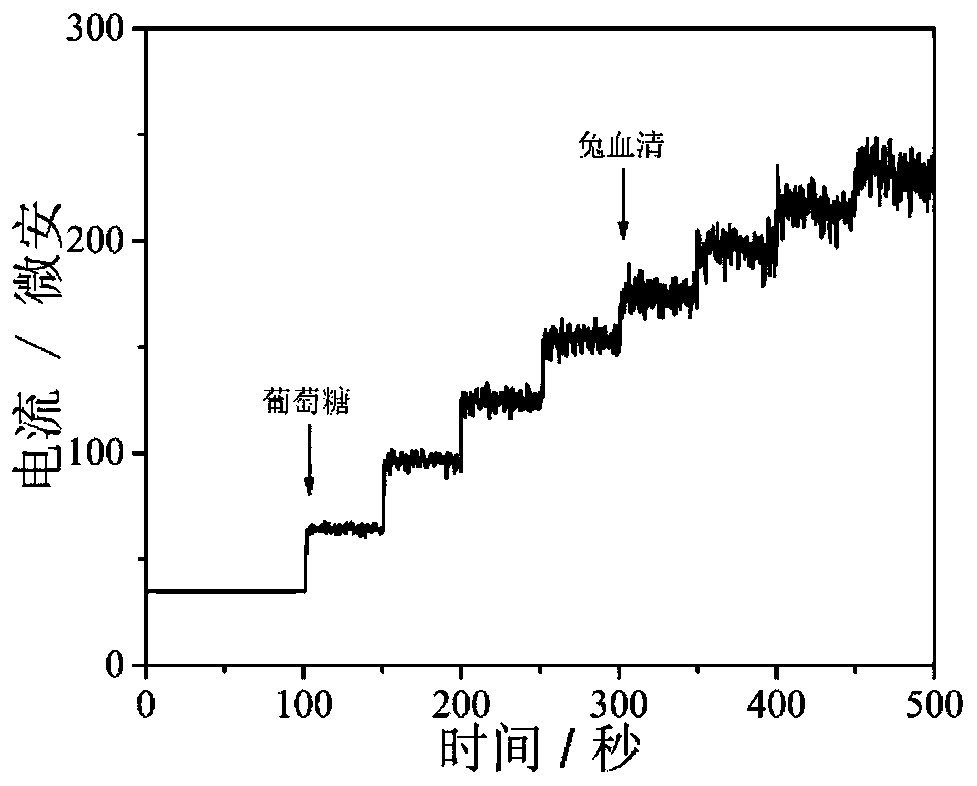
Core-shell hollow Cu(OH)2@Au@Co(OH)2 composite material as well as preparation method and application thereof
A composite material and core-shell technology, applied in analytical materials, chemical instruments and methods, material analysis through electromagnetic means, etc., can solve the problems of cumbersome enzyme protein synthesis process, general enzyme sensing stability, and reduced accuracy. Accelerated electron transfer rate, short charge-sensing path, and improved performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Prepare 20mM K 3 Fe(CN) 6 and 20mM CoCl 2 The aqueous solution was simultaneously injected into the reaction vessel at a rate of 600 μL / min to generate Co 2 [Fe(CN) 6 ] 3 ; Prepare 1mM HAuCl 4 aqueous solution and injected Co at a rate of 300 μL / min 2 [Fe(CN) 6 ] 3 solution, stirred for 30min; then prepared 7mM C 6 h 8 o 6 aqueous solution and added reduced HAuCl at a rate of 500 μL / min 4 , generating Au@Co 2 [Fe(CN) 6 ] 3 , centrifuge the solution at a centrifugal rate of 3000r / min for 10min and redisperse it in 30mL volume water;
[0045] (2) Prepare 20mM K 3 Fe(CN) 6 and 20mM CuCl 2 Aqueous solution was simultaneously injected into Au@Co at a rate of 300 μL / min 2 [Fe(CN) 6 ] 3 In the aqueous solution, centrifuge the solution at a centrifugal rate of 3000r / min for 10min and redisperse in 30mL ethanol solution;
[0046] (3) Prepare 0.4M NaOH aqueous solution and add 20mL to the above ethanol solution, and obtain Cu(OH) after ultrasonication for...
Embodiment 2
[0050] (1) Prepare 5mM K 3 Fe(CN) 6 and 5mM CoCl 2 The aqueous solution was simultaneously injected into the reaction vessel at a rate of 500 μL / min to generate Co 2 [Fe(CN) 6 ] 3 ; Prepare 5mM HAuCl 4 aqueous solution and injected Co at a rate of 200 μL / min 2 [Fe(CN) 6 ] 3 solution, stirred for 30min; then prepared 35mM C 6 h 8 o 6 aqueous solution and added reduced HAuCl at a rate of 400 μL / min 4 , generating Au@Co 2 [Fe(CN) 6 ] 3 , centrifuge the solution at a centrifugal rate of 5000r / min for 15min and redisperse it in 40mL volume water;
[0051] (2) Prepare 5mM K 3 Fe(CN) 6 and 5mM CuCl 2 Aqueous solution was simultaneously injected into Au@Co at a rate of 200 μL / min 2 [Fe(CN) 6 ] 3 In the aqueous solution, centrifuge the solution at a centrifugal rate of 5000r / min for 15min and redisperse in 40mL ethanol solution;
[0052] (3) Prepare 0.8M NaOH aqueous solution and add 30mL to the above ethanol solution, and obtain Cu(OH) after ultrasonication for 30...
Embodiment 3
[0056] (1) Prepare 40mM K 3 Fe(CN) 6 and 40mM CoCl 2 The aqueous solution was simultaneously injected into the reaction vessel at a rate of 600 μL / min to generate Co 2 [Fe(CN) 6 ] 3 ; Prepare 1mM HAuCl 4 aqueous solution and injected Co at a rate of 400 μL / min 2 [Fe(CN) 6 ] 3 solution, stirred for 30min; then prepared 7mM C 6 h 8 o 6 aqueous solution and added reduced HAuCl at a rate of 500 μL / min 4 , generating Au@Co 2 [Fe(CN) 6 ] 3 , centrifuge the solution at a centrifugal rate of 3000r / min for 10min and redisperse it in 60mL volume water;
[0057] (2) Prepare 20mM K 3 Fe(CN) 6 and 20mM CuCl 2 Aqueous solution was simultaneously injected into Au@Co at a rate of 300 μL / min 2 [Fe(CN) 6 ] 3 In the aqueous solution, centrifuge the solution at a centrifugal rate of 3000r / min for 10min and redisperse in 30mL ethanol solution;
[0058] (3) Prepare 0.4M NaOH aqueous solution and add 40mL to the above ethanol solution, and obtain Cu(OH) after ultrasonication for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Limit | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com