Ratiometric fluorescent probe for detecting sulfur dioxide derivatives and application thereof
A ratiometric fluorescent probe, sulfur dioxide technology, applied in fluorescence/phosphorescence, organic chemistry, luminescent materials, etc., to achieve a targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (E)-2-(3-cyano-5,5-dimethyl-4-(4-(piperazine-1-)styrene)furan-2(5H)-ylidene)malononitrile ( 10mmol, 3.73g) was dissolved in dry dichloromethane (30mL), dry triethylamine (12mmol, 1.22g) was added, then chloroacetyl chloride (12mmol, 1.34g) was added dropwise under ice-bath conditions, and the reaction The time is 1h. After the reaction, the mixture was washed 5 times with saturated sodium bicarbonate solution (300 mL), and the organic phase was dried for 2 h by adding an appropriate amount of anhydrous sodium sulfate. After filtration and concentration, Intermediate 1 was obtained by column chromatography.
[0028] 3-(Pyridin-4-yl)-6-(N,N-diethyl)-coumarin (10.5mmol, 147.57mg) and intermediate 1 (0.5mmol, 224.5mg) were dissolved in acetonitrile (10mL) , Potassium iodide (0.6mmol, 99.6mg) was added, and the reaction time of reflux was about 16h. After the temperature was cooled to room temperature, precipitation occurred.
[0029] The obtained precipitate is the ratiom...
Embodiment 2
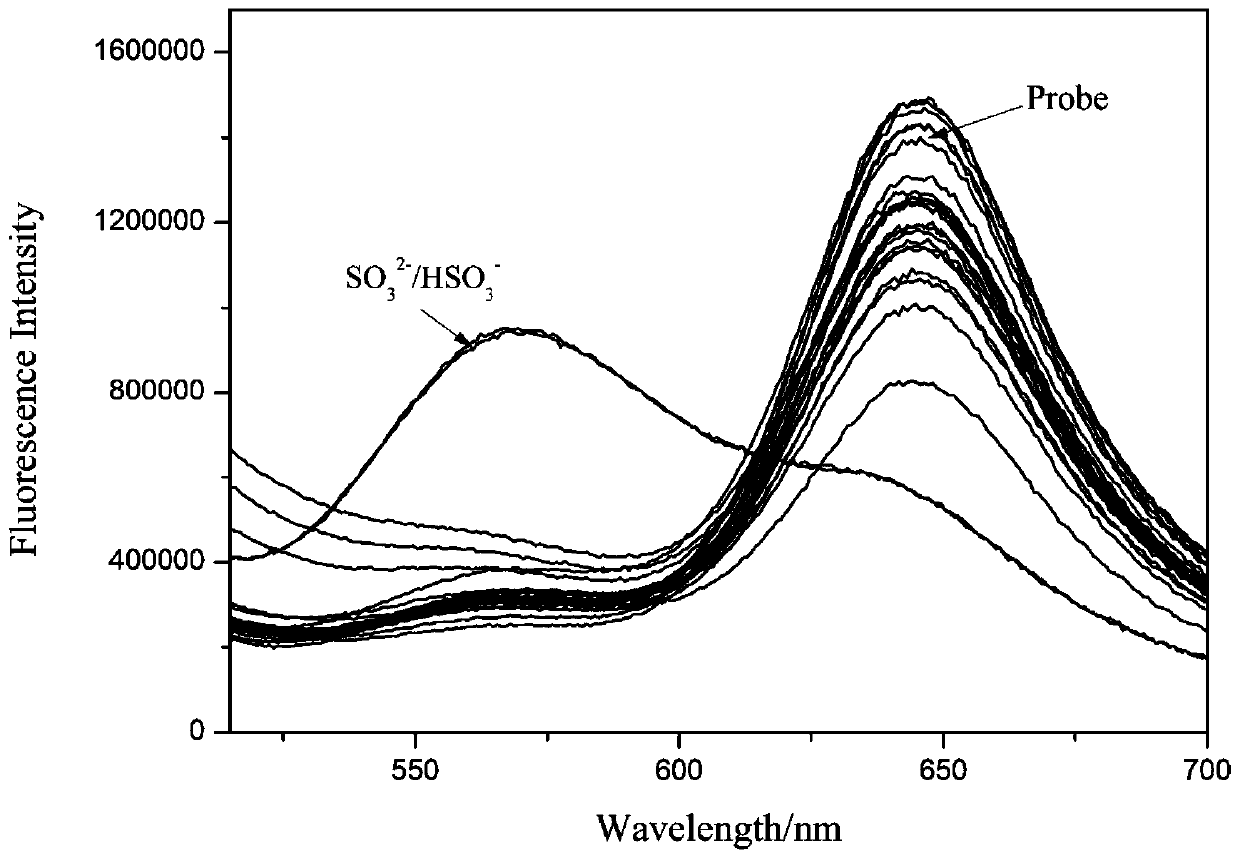
[0034] In a 10mL volumetric flask, prepare a ratio fluorescent probe with a concentration of 5 μM for detecting sulfur dioxide derivatives according to the present invention, and add 10 equivalents of ions and biological thiols, or 1 equivalent of SO 3 2- / HSO 3 - , Fluorescence test was performed after 15 minutes of action.
[0035] The results show that the probe of the present invention is only for SO 3 2- / HSO 3 - have better responsiveness and selectivity, see figure 1 .
Embodiment 3
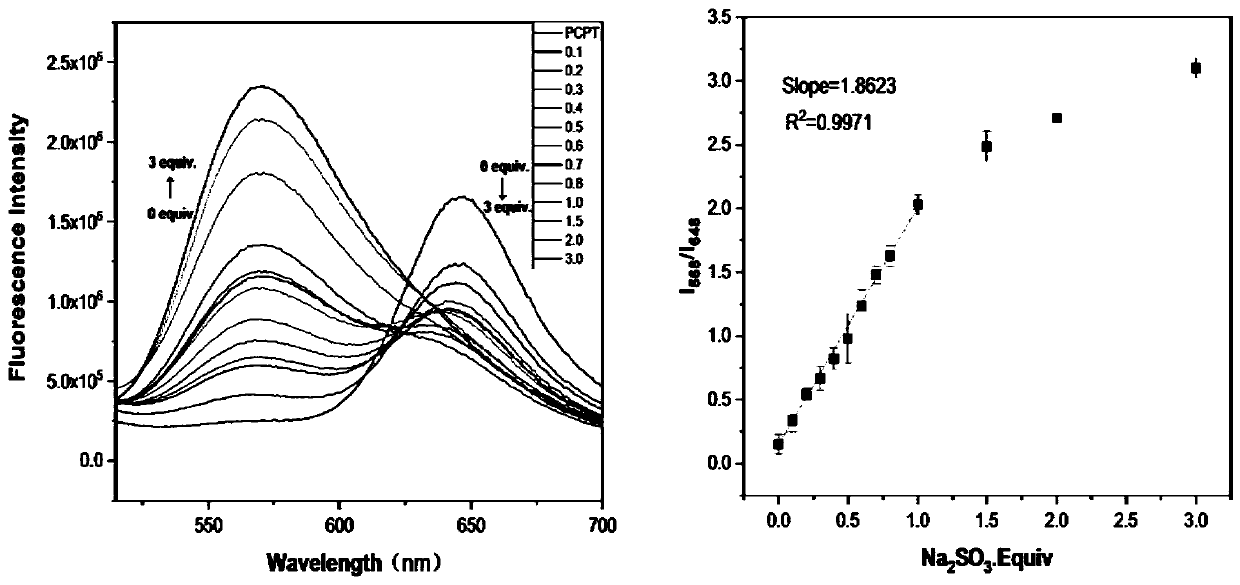
[0037] In a 10mL volumetric flask, prepare a ratio fluorescent probe whose concentration is 5 μM to detect sulfur dioxide derivatives according to the present invention, and add different equivalents of SO with a micro-injector. 3 2- , Fluorescence test was performed after 15 minutes of action.
[0038] The results showed that the ratio of the fluorescence intensity at 568nm to the fluorescence intensity at 648nm relative to SO 3 2- The concentration has a linear relationship within a certain range, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com