siRNA for inhibiting HMGB1 gene, stable nucleic acid lipid nanoparticle containing siRNA, and application of siRNA and stable nucleic acid lipid nanoparticle
A lipid nanoparticle, stable technology, applied in the field of medicine, to achieve the effects of improving utilization rate, inflammation and remission of bullous steatosis, and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
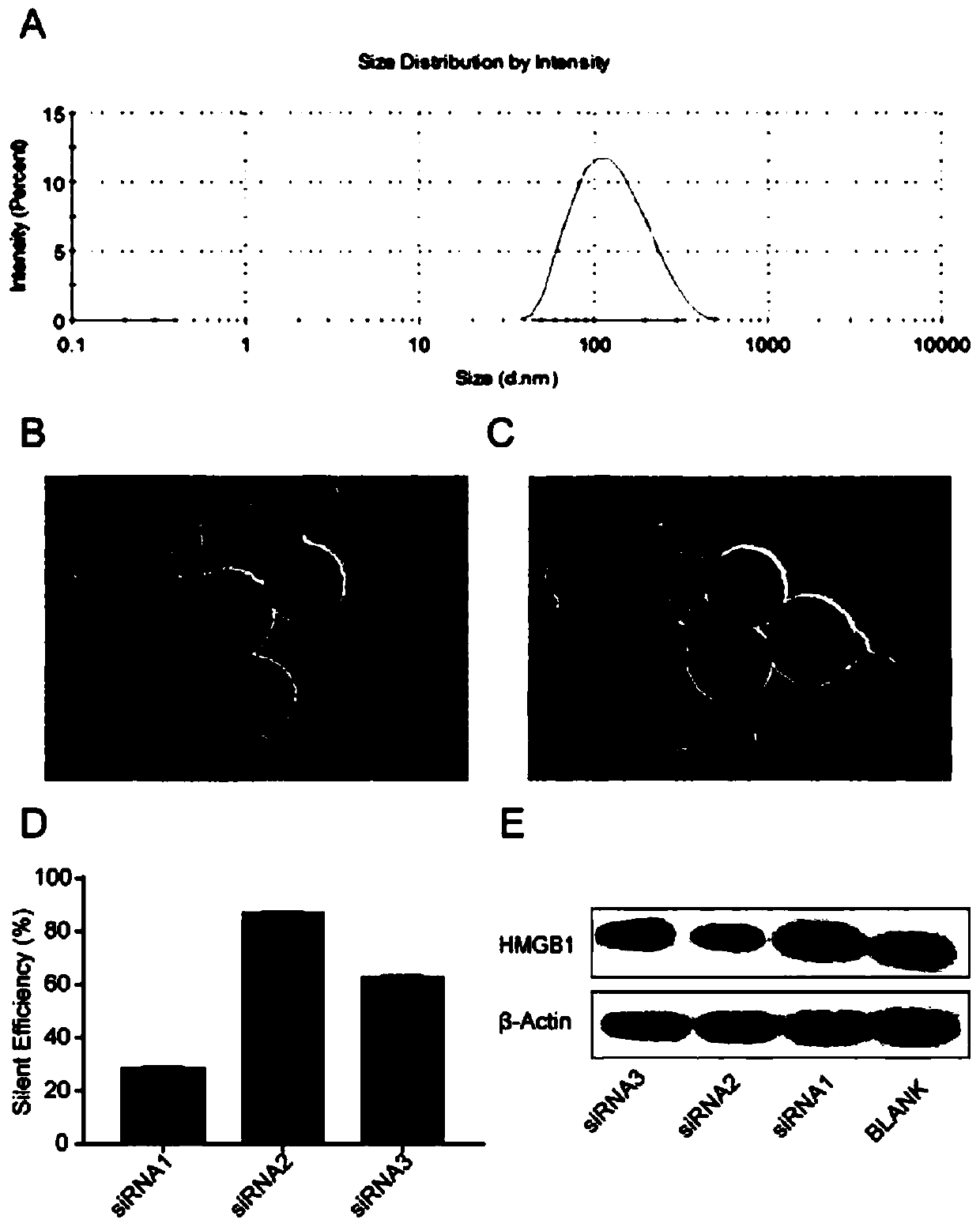
[0057] Example 1 Screening of siRNA Effective Sequence and Preparation and Characterization of Stable Nucleic Acid Lipid Nanoparticles
[0058] Specific steps are as follows:
[0059] 1.1 Design and synthesis of siRNA
[0060] According to the cDNA sequence of mouse HMGB1 gene provided by GenBank, the inventor firstly designed 3 pairs of siRNA sequences targeting HMGB1 with a length of 21 nucleotides using the online design siRNA software, which were respectively denoted as HMGB1 siRNA-1, HMGB1 siRNA-2 and HMGB1 siRNA-3.
[0061] The sequences of HMGB1 siRNA-1, HMGB1 siRNA-2, and HMGB1 siRNA-3 are as follows:
[0062]
[0063] 1.2 Screening of effective siRNA sequences
[0064] RAW246.7 cells were cultured with RPMI-1640 medium, and the cells were collected and counted after trypsinization, and the cells were divided into 6×10 5 Each well was inoculated in a six-well plate with 2 mL of culture medium per well. Transfection was started when the cell density reached 30%-...
Embodiment 2
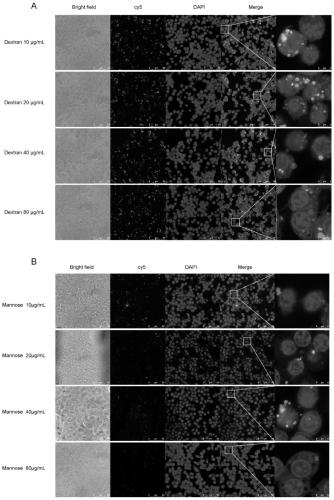
[0084] Example 2 Mannose receptor-mediated targeting of macrophages and investigation of targeting effects in vivo
[0085] Specific steps are as follows:
[0086] 2.1 Receptor blocking experiment: co-incubate cells with different concentrations of dextran or mannose, then add HMGB1-siRNA-cy5@SNALP-Man, and then use confocal laser to observe the amount of fluorescence in the cells to investigate the glucan Fluorescence differences in cells of the sugar and mannose groups. The preparation method of HMGB1-siRNA-cy5@SNALP-Man is the same as the preparation method of HMGB1-siRNA@SNALP-Mannose in Example 1, the difference is that the cy5 fluorescent dye is modified at the end of the siRNA.
[0087] The result is as figure 2 As shown, the dextran group ( figure 2 A) Cell fluorescence uptake did not change significantly with the increase of dextran concentration, but the mannose group ( figure 2 B) As the concentration of mannose increases, the intracellular fluorescence gradu...
Embodiment 3
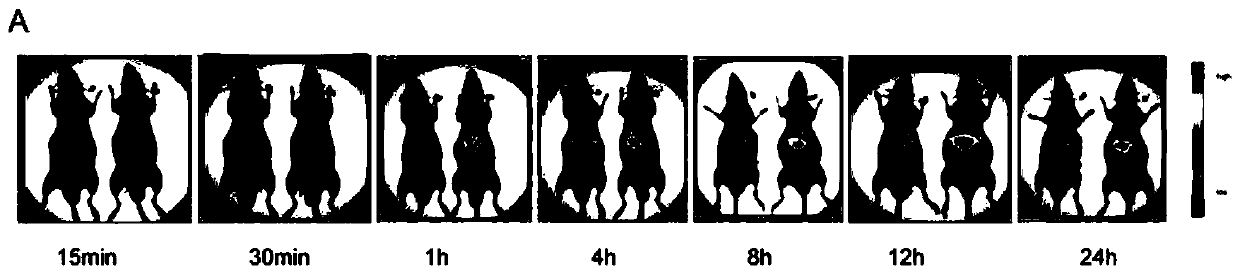
[0089] Example 3 Investigation of HMGB1 silencing effect in vivo
[0090] In order to investigate the effect of silencing the expression of HMGB1 protein and controlling diet on NASH disease, and whether modifying mannose on the surface of the carrier will improve the drug utilization and enhance the therapeutic effect.
[0091] The experimental animals were divided into five groups, namely PBS group, Control-siRNA@SNALP, HMGB1-siRNA@SNALP, HMGB1-siRNA@SNALP-Mannose and HMGB1-siRNA@SNALP-Mannose+NFD group, (NFD is NORMALfeeding diets, that is, the normal feed group).
[0092] The mice in each group were injected into the tail vein twice a week. At the same time, they were fed with high-fat diet and normal diet according to the experimental design. After 8 times of administration, the mice were dissected and their livers were taken for QPCR experiments and Western Blot. Experiments, HE staining and immunohistochemical experiments. The differences in the expression of HMGB1 am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com