Method for producing aromatic hydrocarbon from methanol
A technology for methanol and aromatics, applied in the production of aromatics and the production of aromatics from methanol, can solve the problems of reducing the conversion rate of methanol to aromatics, reducing the aromatization reaction, and being unfavorable for the aromatization reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
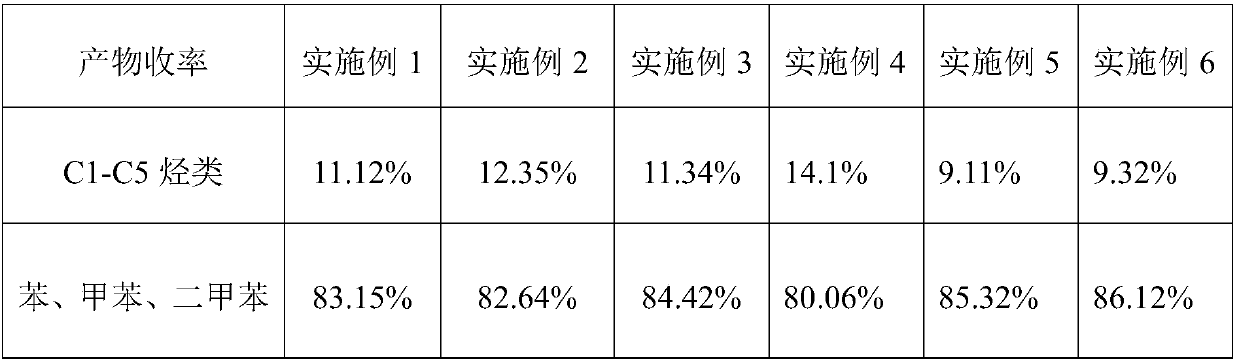
Examples
Embodiment 1
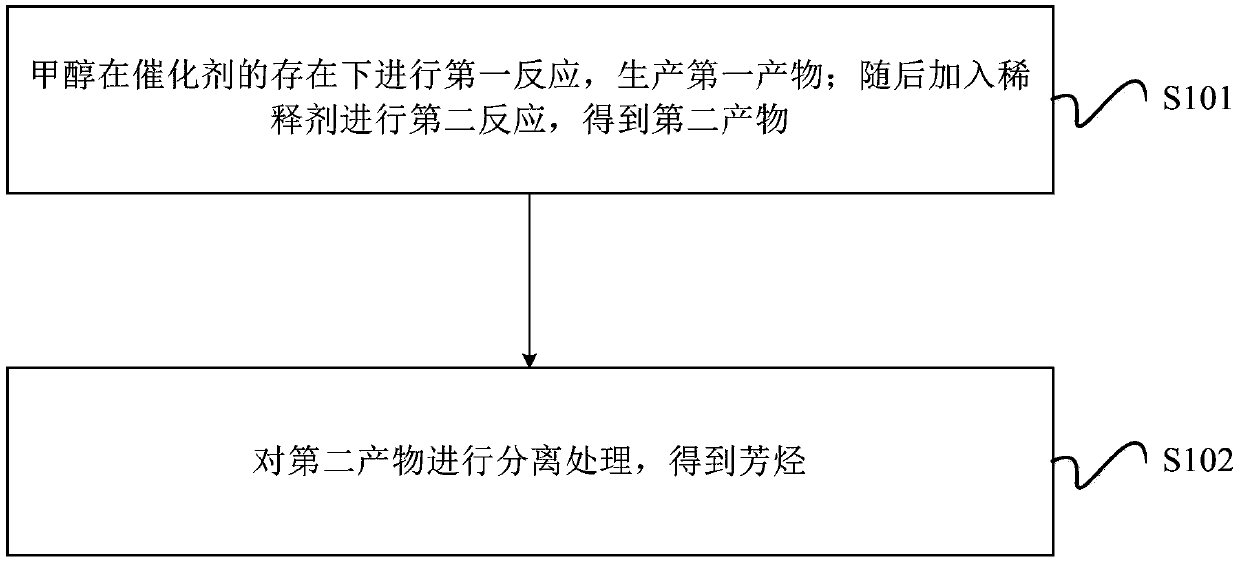
[0070] The method for producing aromatics from methanol in this embodiment includes the following steps:
[0071] 1) Formation of the first product
[0072] Raw material for the first reaction: methanol
[0073] Catalyst (carbon content less than 0.5%): ZSM-5 molecular sieve modified with zinc, and the mass fraction of zinc in ZSM-5 molecular sieve is 1%
[0074] The first reactor: a moving bed reactor
[0075] Specifically, the methanol and the catalyst are mixed in a moving bed reactor and then the first reaction is performed to generate the first product.
[0076] The temperature of the first reaction is 250°C, the pressure is 0.5Mpa, and the space velocity of methanol is 1 / h.
[0077] Among them, the first product includes dimethyl ether, water produced by methanol cracking, C1-C5 hydrocarbons, C6+ other hydrocarbons, and unreacted methanol.
[0078] In the first product, the mass fraction of dimethyl ether is 80%.
[0079] In the first product, the mass fraction of methanol is 20%.
[0...
Embodiment 2
[0092] The method for producing aromatics from methanol in this embodiment includes the following steps:
[0093] 1) Formation of the first product
[0094] Raw material for the first reaction: methanol
[0095] Catalyst (carbon content less than 0.5%): ZSM-5 molecular sieve modified with zinc and gallium, and the mass fraction of zinc in ZSM-5 molecular sieve is 2%, and the mass of gallium in ZSM-5 molecular sieve The score is 1%
[0096] The first reactor: a moving bed reactor
[0097] Specifically, the methanol and the catalyst are mixed in a moving bed reactor and then the first reaction is performed to generate the first product.
[0098] The temperature of the first reaction is 220°C, the pressure is 0.5Mpa, and the space velocity of methanol is 2 / h.
[0099] Among them, the first product includes dimethyl ether, water produced by methanol cracking, C1-C5 hydrocarbons, C6+ other hydrocarbons, and unreacted methanol.
[0100] In the first product, the mass fraction of dimethyl ether ...
Embodiment 3
[0114] The method for producing aromatics from methanol in this embodiment includes the following steps:
[0115] 1) Formation of the first product
[0116] Raw material for the first reaction: methanol
[0117] Catalyst (carbon content less than 0.5%): ZSM-5 molecular sieve modified with silver and boron, and the mass fraction of silver in ZSM-5 molecular sieve is 1%, and the mass of boron in ZSM-5 molecular sieve The score is 5%
[0118] The first reactor: a moving bed reactor
[0119] Specifically, the methanol and the catalyst are mixed in a moving bed reactor and then the first reaction is performed to generate the first product.
[0120] The temperature of the first reaction is 270°C, the pressure is 2Mpa, and the space velocity of methanol is 4 / h.
[0121] Among them, the first product includes dimethyl ether, water produced by methanol cracking, C1-C5 hydrocarbons, C6+ other hydrocarbons, and unreacted methanol.
[0122] In the first product, the mass fraction of dimethyl ether is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com