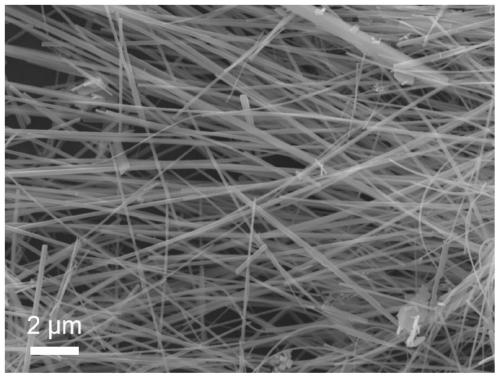
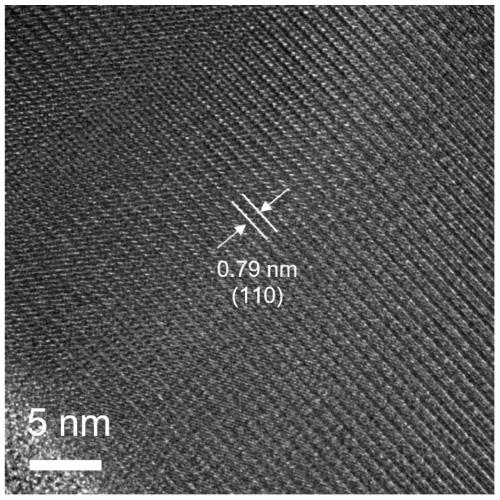
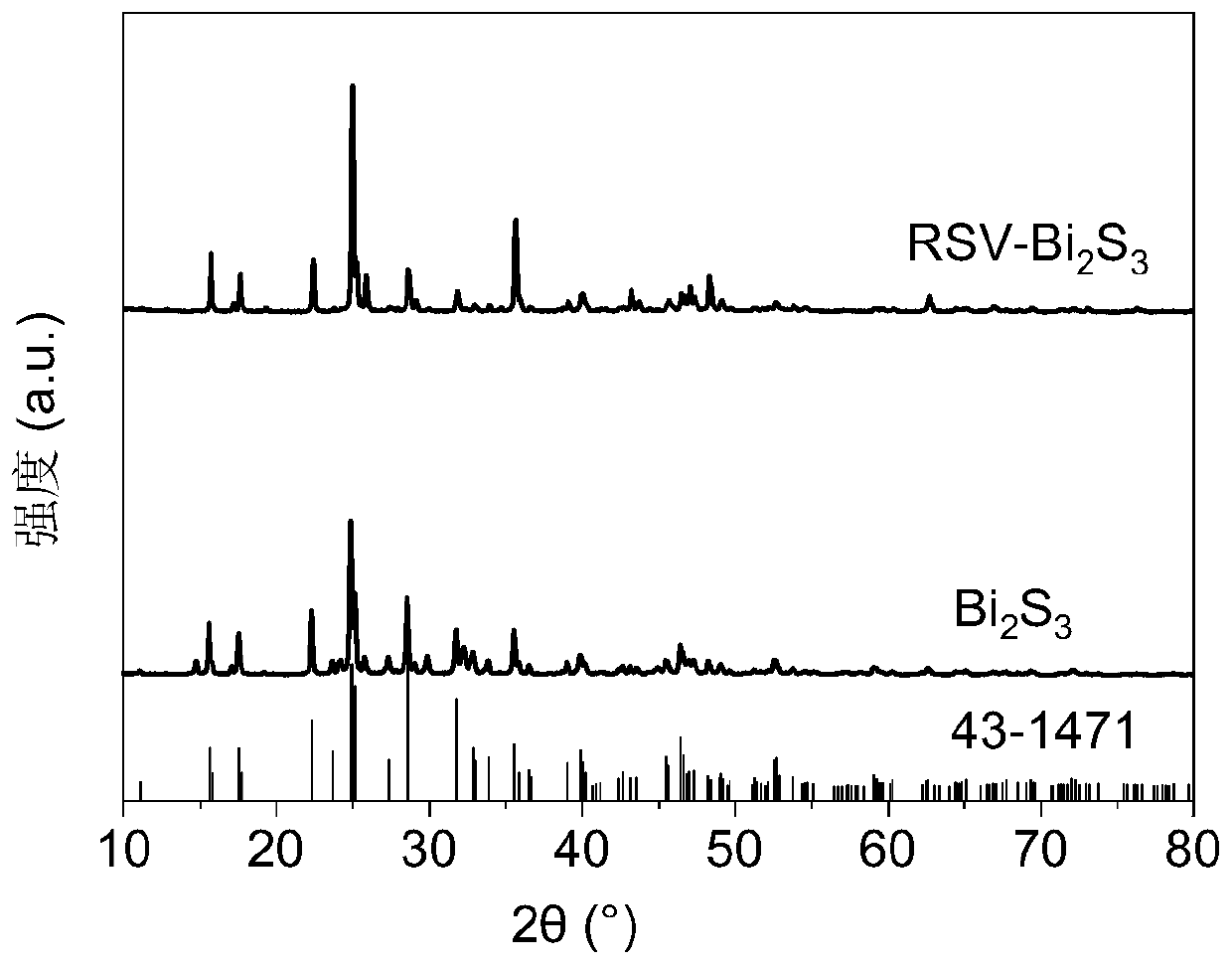
Sulfur-rich vacancy bismuth sulfide nanowire as well as preparation method and application thereof
A bismuth sulfide and nanowire technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problem of low selectivity of formic acid products, achieve mild conditions, simple process, and control material selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] RSV-Bi 2 S 3 Nanowire preparation method, comprising the following steps:
[0033] 1) Add 1.6g sodium sulfide nonahydrate, 0.8g bismuth nitrate pentahydrate, 5g lithium hydroxide, and 10g potassium nitrate into a 100ml reaction kettle;
[0034] 2) Fully shake and oscillate to mix the reactants evenly, then add 5ml of deionized water, and put it in an oven at 120°C for 72 hours;
[0035] 3) After washing the obtained product with deionized water for 3-4 times, put it into a 70°C oven and dry it to obtain black bismuth sulfide solid nanowires;
[0036] 4) Add 35.2g of ascorbic acid to 200ml of deionized water to prepare a 1mol / L ascorbic acid solution;
[0037] 5) Add 200 mg of the bismuth sulfide obtained in step 3), and stir for 3 hours at a rotational speed of 300 r / min; 6) Wash the product with deionized water for 3-4 times, and dry it in an oven at 70°C to obtain RSV-Bi 2 S 3 Nanowires.
[0038] Take the RSV-Bi of this example 2 S 3 Taking nanowires as an exa...
Embodiment 2
[0044] RSV-Bi 2 S 3 Nanowire preparation method, comprising the following steps:
[0045] 1) Add 1.6g sodium sulfide nonahydrate, 0.8g bismuth nitrate pentahydrate, 5g lithium hydroxide, and 10g potassium nitrate into a 100ml reaction kettle;
[0046] 2) Fully shake and oscillate to mix the reactants evenly, then add 5ml of deionized water, and put it in an oven at 120°C for 72 hours;
[0047] 3) After washing the obtained product with deionized water for 3-4 times, put it into a 70° C. oven and dry it to obtain black bismuth sulfide nanowires;
[0048] 4) Add 35.2g of ascorbic acid to 200ml of deionized water to prepare a 1mol / L ascorbic acid solution;
[0049] 5) Add 200 mg of the bismuth sulfide obtained in step 3), and stir for 3 hours at a rotational speed of 350 r / min; 5) Wash the product with deionized water for 3-4 times, and dry it in an oven at 70°C to obtain RSV-Bi 2 S 3 Nanowires.
[0050] With the product RSV-Bi of the present invention 2 S 3 Taking nanowi...
Embodiment 3
[0053] RSV-Bi 2 S 3 Nanowire preparation method, comprising the following steps:
[0054] 1) Add 1.6g sodium sulfide nonahydrate, 0.8g bismuth nitrate pentahydrate, 6g lithium hydroxide, and 9g potassium nitrate into a 100ml reaction kettle;
[0055] 2) Fully shake and oscillate to mix the reactants evenly, then add 5ml of deionized water, and put it in an oven at 120°C for 72 hours;
[0056] 3) After washing the obtained product with deionized water for 3-4 times, put it into a 70° C. oven and dry it to obtain black bismuth sulfide nanowires;
[0057] 4) Add 35.2g of ascorbic acid to 200ml of deionized water to prepare a 1mol / L ascorbic acid solution;
[0058] 5) Add 300mg of bismuth sulfide obtained in step 3), and stir for 3h at a speed of 300r / min;
[0059] 5) After washing the product with deionized water for 3-4 times, put it in a 70°C oven to dry to obtain RSV-Bi 2 S 3 Nanowires.
[0060] With the product RSV-Bi of the present invention 2 S 3 Taking nanowires a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com