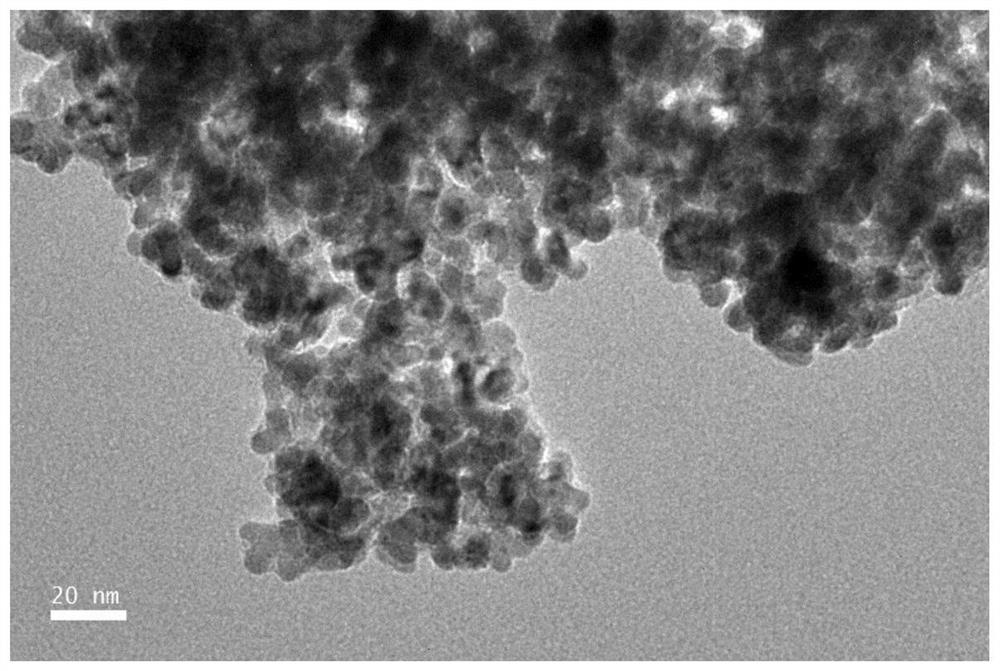
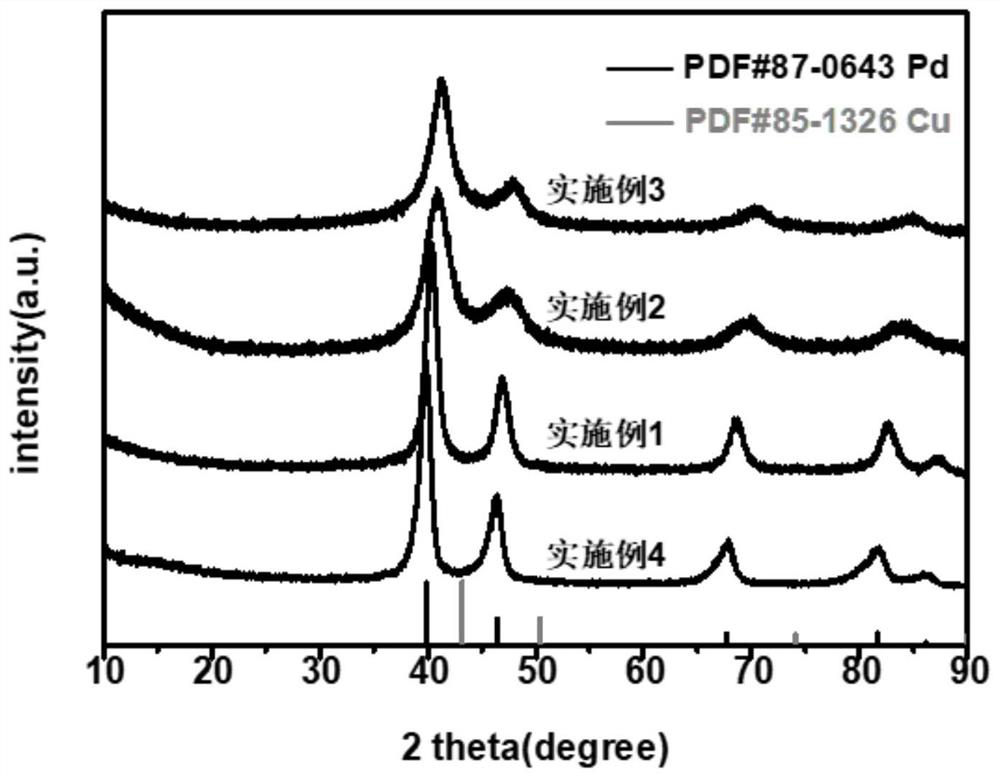
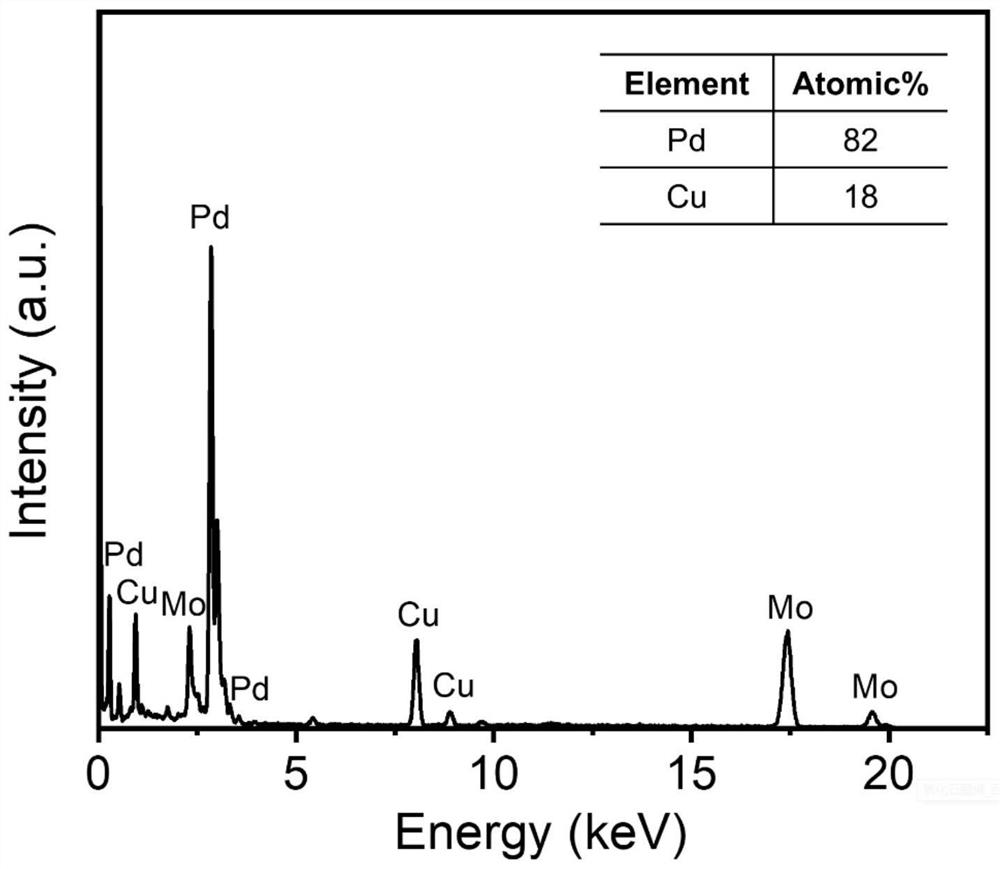
Carbon dioxide electroreduction catalyst with high formic acid selectivity and preparation method thereof
A carbon dioxide and catalyst technology, applied in the field of carbon dioxide electroreduction catalyst and its preparation, can solve the problems of poor selectivity, small potential window, high overpotential, etc., and achieve the effect of reducing energy consumption and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Preparation of template precursor: 0.0783g (0.24mmol) potassium tetrachloropalladate was dissolved in 12mL water, 0.01g (0.06mmol) copper chloride dihydrate was dissolved in 3mL water, then the two were mixed evenly, and Add 15mL (0.83mol) of water and 200mg (0.015mmol) of polyether F127 (molecular weight is about 13300), and stir at 1000rpm for 30min to completely dissolve the polyether F127.
[0057] (2) Preparation of reducing agent: 0.309 g (1.75 mmol) of ascorbic acid was dissolved in 17.5 mL (0.97 mol) of water to obtain an S2 solution.
[0058] (3) Synthesis of palladium-copper alloy: under the condition of 95° C. in an oil bath, the above-mentioned S2 solution was added dropwise to the S1 solution, and reacted for 3 hours. After the reaction was completed, the product was collected by centrifugation (the centrifugal speed was 9000r / min, and the time was 6min). And washed three times with a mixed solution of ethanol and water with a volume ratio of 3:2 (ethan...
Embodiment 2
[0060] (1) Preparation of template precursor: Dissolve 0.0588g (0.18mmol) potassium tetrachloropalladate in 9mL water, 0.02g (0.12mmol) copper chloride dihydrate in 6mL water, then mix the two evenly, and add Add 15mL of water and 200mg of polyether F127 (molecular weight is about 13300), and stir at 1000rpm for 30min to completely dissolve the polyether F127, which is the S1 solution, ready for use.
[0061] (2) Preparation of reducing agent: 0.309g of ascorbic acid was dissolved in 17.5mL of water to obtain S2 solution.
[0062] (3) Synthesis of palladium-copper alloy: under the condition of 95° C. in an oil bath, the above-mentioned S2 solution was added dropwise to the S1 solution, and reacted for 3 hours. After the reaction was completed, the product was collected by centrifugation (the centrifugal speed was 9000r / min, and the time was 6min). And washed three times with a mixed solution of ethanol and water with a volume ratio of 3:2 (ethanol 15mL, water 10mL), and the wa...
Embodiment 3
[0064] (1) Preparation of template precursor: 0.049g (0.15mmol) potassium tetrachloropalladate was dissolved in 7.5mL water, 0.0256g (0.15mmol) copper chloride dihydrate was dissolved in 7.5mL water, and then the two were mixed evenly, Add 15mL of water and 200mg of polyether F127 (molecular weight is about 13300) to it, stir at 1000rpm for 30min to completely dissolve the polyether F127, and obtain the S1 solution for use.
[0065] (2) Preparation of reducing agent: 0.309g of ascorbic acid was dissolved in 17.5mL of water to obtain S2 solution.
[0066] (3) Synthesis of palladium-copper alloy: under the condition of 95° C. in an oil bath, the above-mentioned S2 solution was added dropwise to the S1 solution, and reacted for 3 hours. After the reaction was completed, the product was collected by centrifugation (the centrifugal speed was 9000r / min, and the time was 6min). And washed three times with a mixed solution of ethanol and water with a volume ratio of 3:2 (ethanol 15mL,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com