Application and preparation method of a perovskite catalyst in catalytic carbon dioxide hydrogenation formic acid
A technology for catalyzing carbon dioxide and carbon dioxide, applied in the preparation of organic compounds, chemical elements of heterogeneous catalysts, preparation of carboxylate, etc., can solve the problems of low TON value, harsh reaction conditions, product decomposition, etc., and achieve long reaction time. , The effect of high reactivity and high TON
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
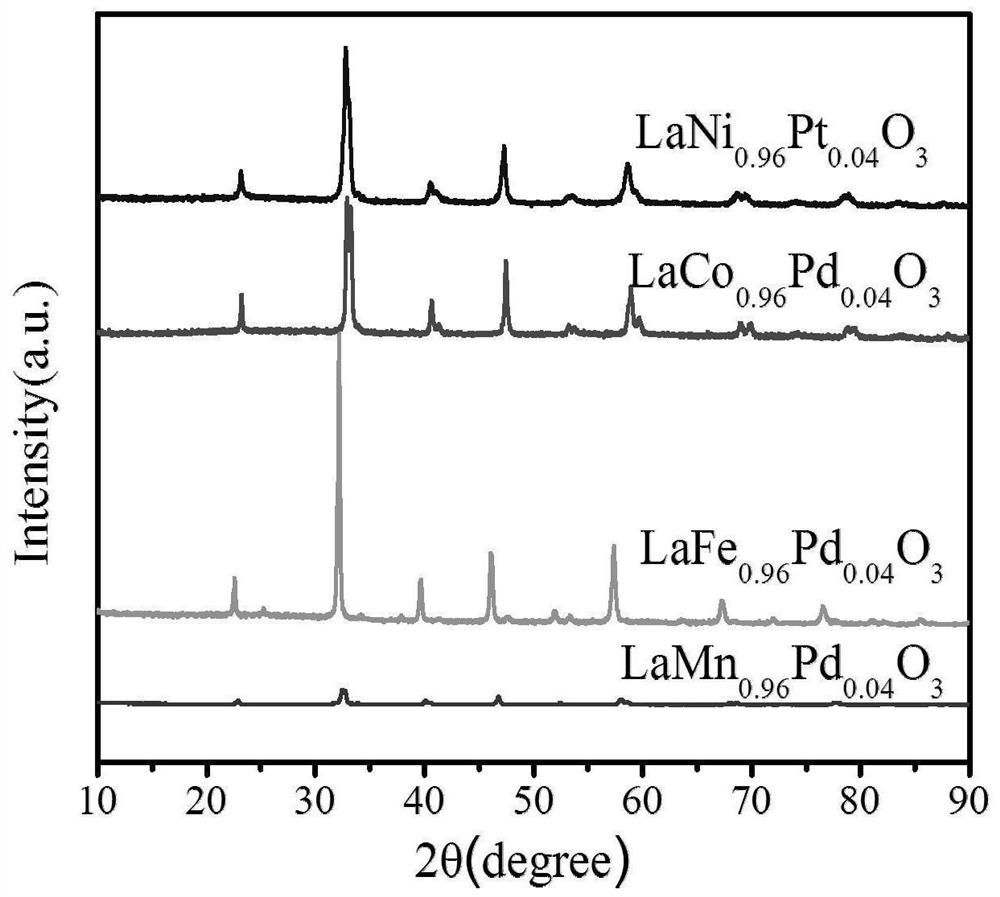
[0027] A perovskite catalyst for catalyzing the hydrogenation of carbon dioxide to formic acid, the A site of the perovskite is La, the B site is Mn and Pd, and the mass fraction of Pd is 1.74wt% of the perovskite.
[0028] The above-mentioned preparation method of the perovskite catalyst for catalyzing carbon dioxide hydrogenation to produce formic acid specifically comprises the following steps:
[0029] Dissolve 1.7320g lanthanum nitrate hexahydrate, 1.5370g anhydrous citric acid, 2.3379g ethylenediaminetetraacetic acid, 0.9639g tetrahydrate manganese nitrate and 0.0426g dihydrate palladium nitrate in 30mL deionized water, stir rapidly until the solid is completely dissolved, Ammonia water was added dropwise to the mixed solution to adjust the pH value of the solution to about 8; at this time, the solution was stirred at 550 r / min, heated at 80 °C, evaporated excess liquid to form a viscous gel, and the magnet was taken out; placed in an oven at 170 Dry at ℃ for 12h, and gr...
Embodiment 2
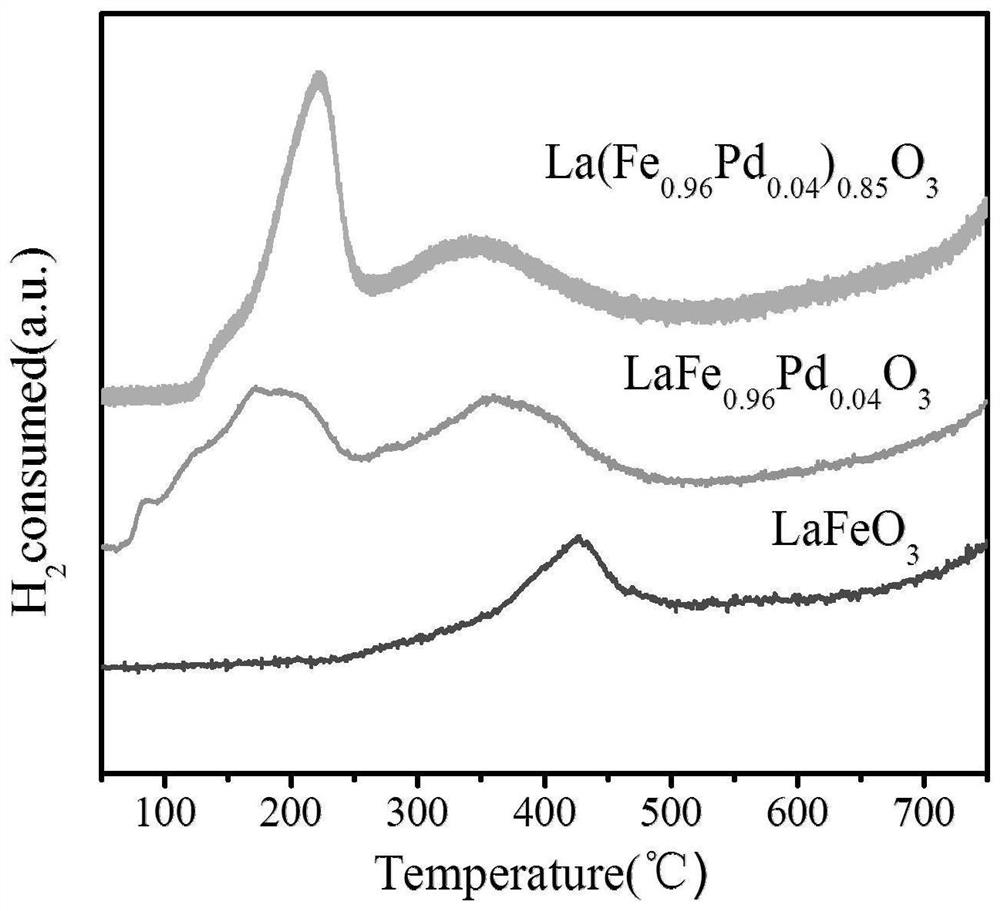
[0032] A perovskite catalyst for catalyzing carbon dioxide hydrogenation to formic acid, the A site of the perovskite is La, the B site is Fe and Pd, and the mass fraction of Pd is 1.74wt%.
[0033] The above-mentioned preparation method of the perovskite catalyst for catalyzing carbon dioxide hydrogenation to produce formic acid specifically comprises the following steps:
[0034] Dissolve 1.7320g lanthanum nitrate hexahydrate, 1.5370g anhydrous citric acid, 2.3379g ethylenediaminetetraacetic acid, 1.5514g nonahydrate ferric nitrate and 0.0426g dihydrate palladium nitrate in 30mL deionized water, stir rapidly until the solid is completely dissolved, Ammonia water was added dropwise to the mixed solution to adjust the pH value of the solution to about 8; at this time, the solution was stirred at 550 r / min, heated at 80 °C, evaporated excess liquid to form a viscous gel, and the magnet was taken out; placed in an oven at 170 Dry at ℃ for 12h, naturally cool and grind to obtain ...
Embodiment 3
[0038] A perovskite catalyst for catalyzing carbon dioxide hydrogenation to formic acid, the A site of the perovskite is La, the B site is Co and Pd, and the mass fraction of Pd is 1.74wt%.
[0039] The above-mentioned preparation method of the perovskite catalyst for catalyzing carbon dioxide hydrogenation to produce formic acid specifically comprises the following steps:
[0040] Dissolve 1.7320g lanthanum nitrate hexahydrate, 1.5370g anhydrous citric acid, 2.3379g ethylenediaminetetraacetic acid, 1.1176g cobalt nitrate hexahydrate and 0.0426g palladium nitrate dihydrate in 30mL deionized water, stir rapidly until the solid is completely dissolved, Ammonia water was added dropwise to the mixed solution to adjust the pH value of the solution to about 8; at this time, the solution was stirred at 550 r / min, heated at 80 °C, the excess liquid was evaporated to form a viscous gel, and the magnet was taken out; placed in an oven, 170 Dry at ℃ for 12h, naturally cool and grind to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com