NiTi nanoflower hydrotalcite photocatalyst as well as preparation method and application thereof
A nanoflower and catalyst technology, applied in the field of NiTi hydrotalcite photocatalyst and its preparation, can solve the problems of unfavorable application, limited reaction conditions, etc., and achieve the advantages of simple preparation method, low requirements on reaction conditions, and improved apparent quantum efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of nickel-titanium nano flower hydrotalcite photocatalyst, comprising the steps of:
[0038] (1) Dissolve 0.29g of nickel nitrate hexahydrate, 0.237g of titanium sulfate and 2.8g of urotropine into 50 mL of deionized water, stir well to obtain a transparent solution;
[0039] (2) Transfer the transparent solution described in step (1) to a polytetrafluoroethylene lining and heat up in a muffle furnace for a heating reaction. The temperature of the heating reaction is 140°C, and the heating reaction time is 9 hours. Cool to room temperature. The heated product was obtained, and the precipitate was collected by suction filtration;
[0040] (3) drying the precipitate in step (2) at 30° C. to obtain the nickel-titanium nanoflower hydrotalcite photocatalyst.
Embodiment 2
[0042] A preparation method of nickel-titanium nano flower hydrotalcite photocatalyst, comprising the steps of:
[0043] (1) Dissolve 0.8721g of nickel nitrate hexahydrate, 0.237g of titanium sulfate and 2.8g of urotropine into 50 mL of deionized water, stir well to obtain a transparent solution;
[0044] (2) Transfer the transparent solution described in step (1) to a polytetrafluoroethylene lining and heat up in a muffle furnace for a heating reaction. The temperature of the heating reaction is 140°C, and the heating reaction time is 9 hours. Cool to room temperature. The heated product was obtained, and the precipitate was collected by suction filtration;
[0045] (3) drying the precipitate in step (2) at 60° C. to obtain the nickel-titanium nanoflower hydrotalcite photocatalyst.
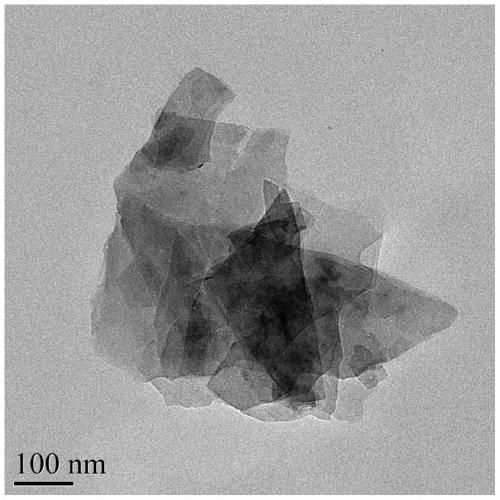
[0046] The effect that the nickel-titanium nano flower hydrotalcite photocatalyst that embodiment 2 makes is observed under the scanning electron microscope is as follows: figure 1 shown, from ...
Embodiment 3
[0048] A preparation method of nickel-titanium nano flower hydrotalcite photocatalyst, comprising the steps of:
[0049] (1) Dissolve 1.45g of nickel nitrate hexahydrate, 0.237g of titanium sulfate and 2.8g of urotropine into 50 mL of deionized water, stir well to obtain a transparent solution;
[0050] (2) Transfer the transparent solution described in step (1) to a polytetrafluoroethylene lining and heat up in a muffle furnace for a heating reaction. The temperature of the heating reaction is 140°C, and the heating reaction time is 9 hours. Cool to room temperature. The heated product was obtained, and the precipitate was collected by suction filtration;
[0051] (3) drying the precipitate in step (2) at 100° C. to obtain the nickel-titanium nanoflower hydrotalcite photocatalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com