Bifunctional pillararene derivative ligand, metal organic cage and preparation method
A dual-functional group, metal-organic technology, applied in the field of supramolecular compound preparation, can solve the problems of MOC with large steric hindrance and difficult to modify functional groups, and achieve the effects of reducing steric hindrance, convenient preparation, and solving technical difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
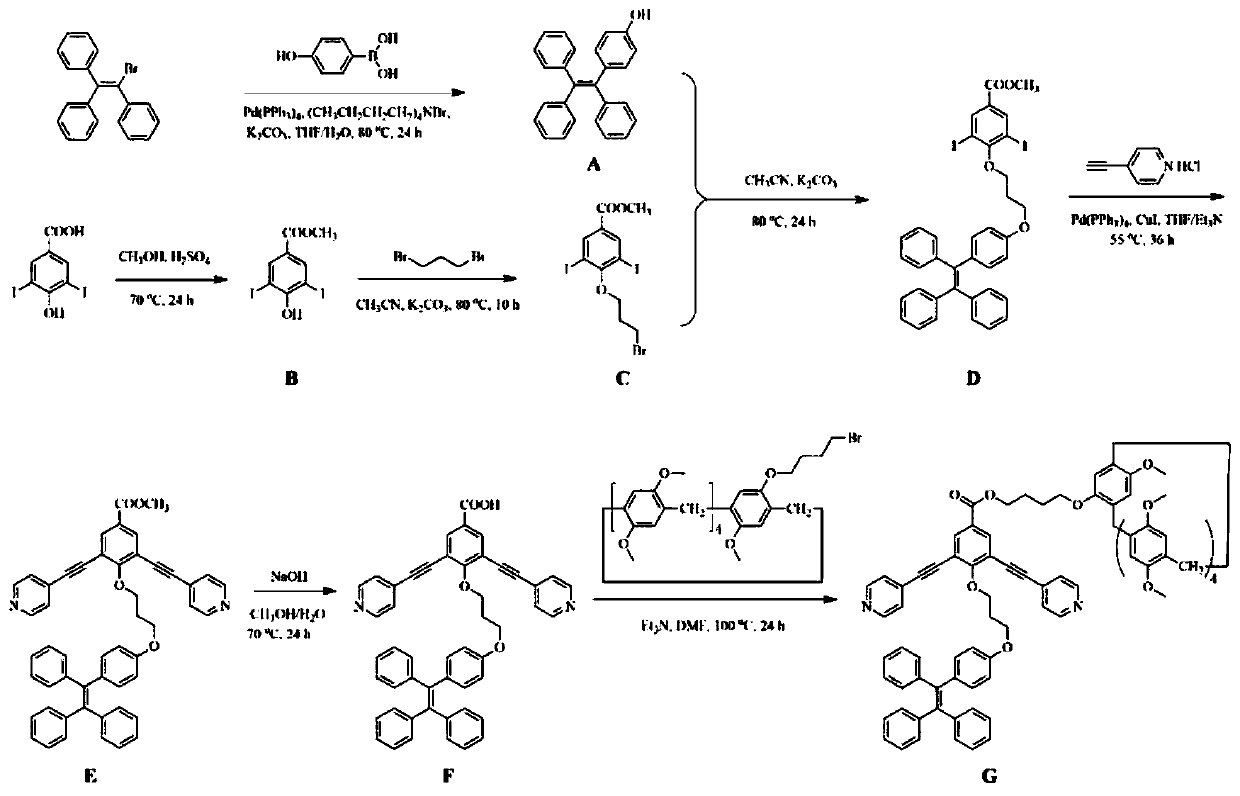
[0038] The synthesis of embodiment 1 compound A-G
[0039](1) In a 500ml Schlenk bottle equipped with a magnetic stirrer, add 2-bromo-1,1,2-triphenylethylene (15g, 44.71mmol), 4-hydroxyphenylboronic acid (10g, 72.46mmol) and tetrabutyl ammonium bromide (44mg, 0.136mmol), slowly add 2mol / L of K 2 CO 3 Aqueous solution (36ml, 72mmol), repeated nitrogen replacement three times to fully remove oxygen, added tetrakis(triphenylphosphine) palladium (258mg, 2.23mmol) under nitrogen protection, introduced 80ml tetrahydrofuran with a needle, reacted at 80°C for 24h, and reduced to Distilled under high pressure, the crude product was extracted with ethyl acetate, using petroleum ether: ethyl acetate = 5:1 as eluent, and column chromatography gave 14.6 g of light yellow solid product with a yield of 94%. use 1 H NMR characterizes its structure, confirming that the light yellow solid is compound A, and its structural formula is 1 H NMR (500MHz, CDCl 3 )δ (ppm): 7.18–7.00 (m, 15H), 6...
Embodiment 2
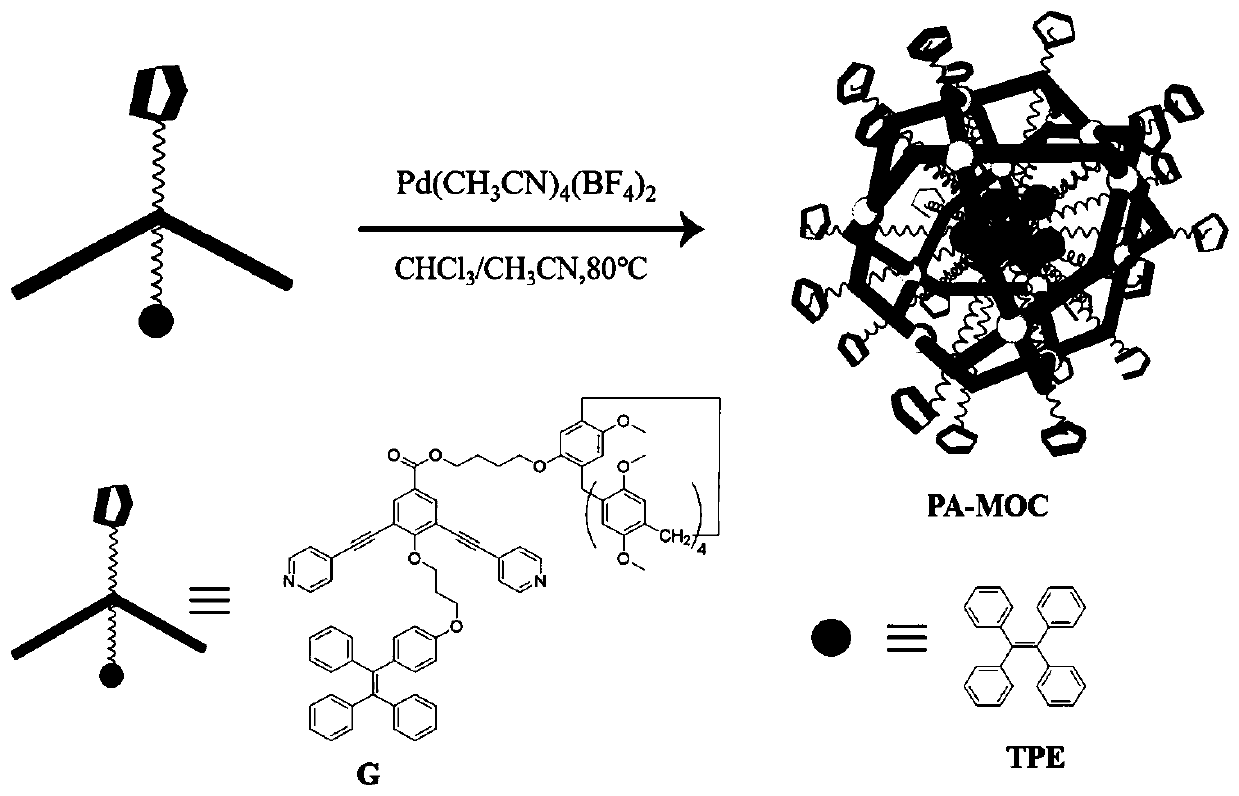
[0046] Example 2 Preparation of bifunctional pillar arene derivative metal organic cage (PA-MOC)
[0047] A method for preparing a metal-organic cage of bifunctional pillar arene derivatives driven by a coordination bond, comprising the following steps:
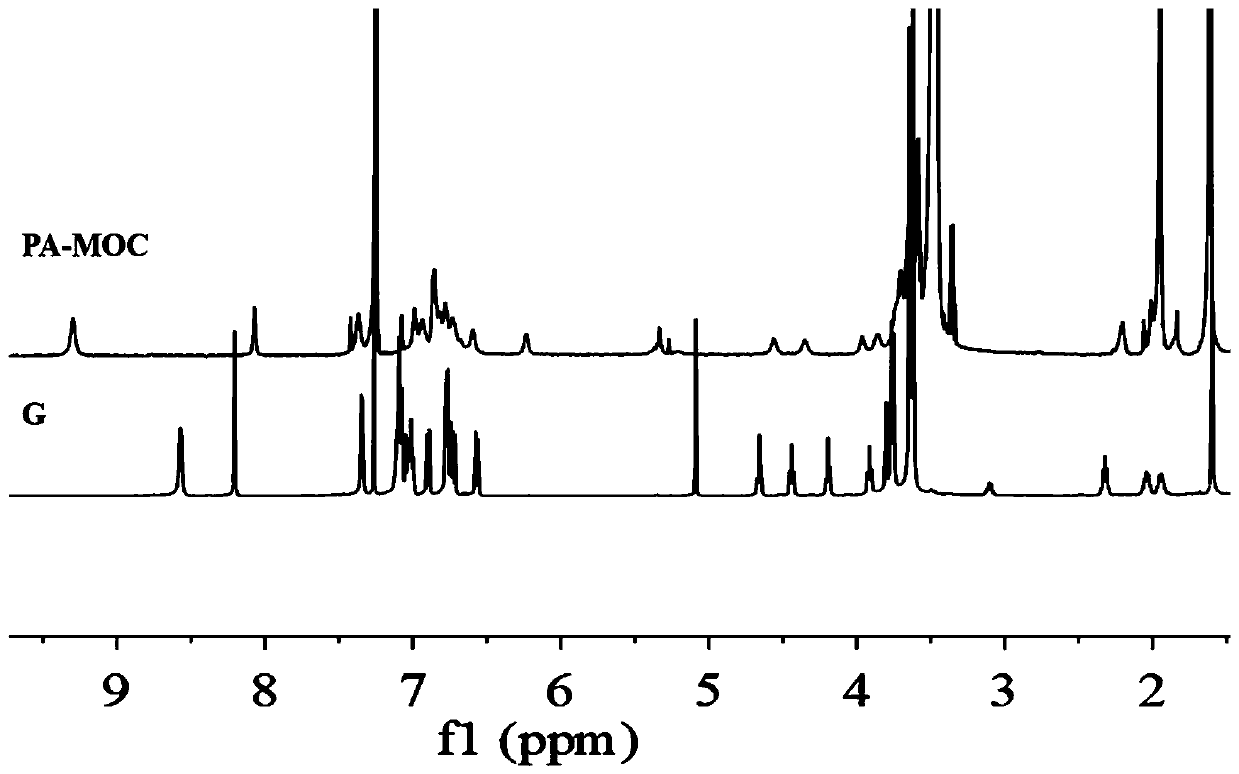
[0048] The synthesized ligand, compound G (7.4 mg, 4.87 μmol) was dissolved in 100 μl chloroform solvent, and Pd(CH 3 EN) 4 (BF 4 ) 2 Acetonitrile solution (15.8mg / ml, 68μl), mixed thoroughly, heated at 80°C for 24h, after the reaction, a dark green homogeneous mother liquor was formed, precipitated in anhydrous ether, and dried in vacuum to obtain a light green solid product, which was again dissolved in CDCl 3 in, through 1 The chemical shift changes of the protons were observed by H NMR, thus confirming the formation of metal-organic cages.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com