Fluorine-containing compound, application, ethylene oligomerization catalyst composition, ethylene oligomerization method, ethylene trimerization method and ethylene tetramerization method
A compound and composition technology, applied in the field of fluorine-containing compounds, can solve the problems of unfavorable process economy and achieve the effects of high catalytic activity, high stability and fast initiation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
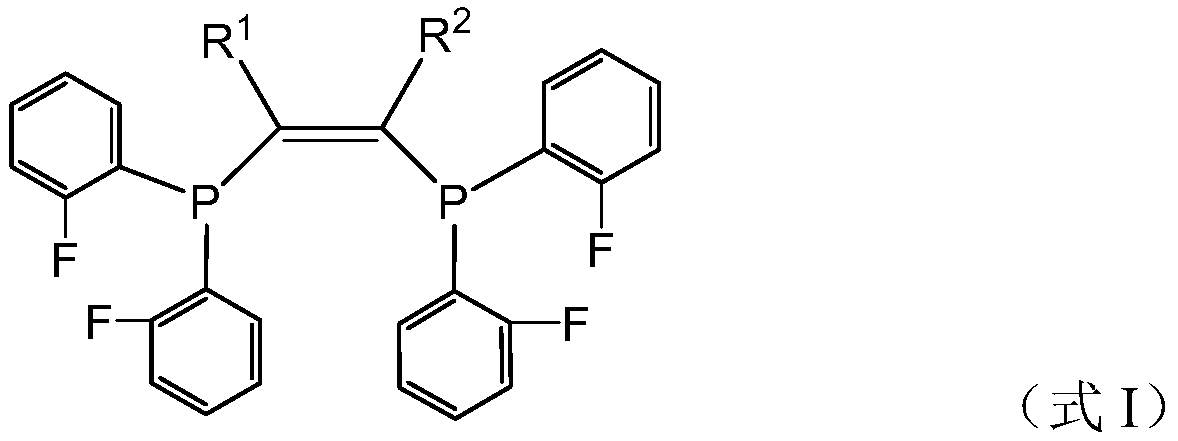
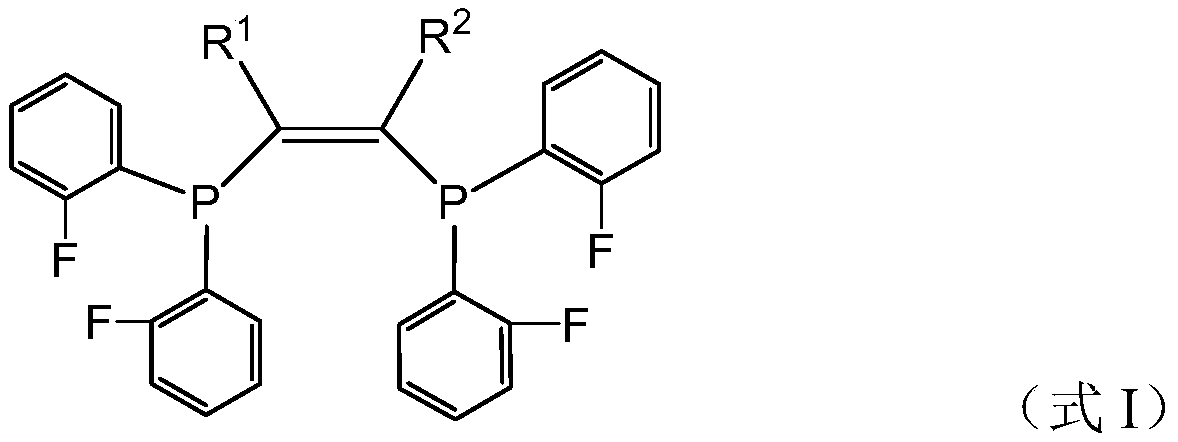
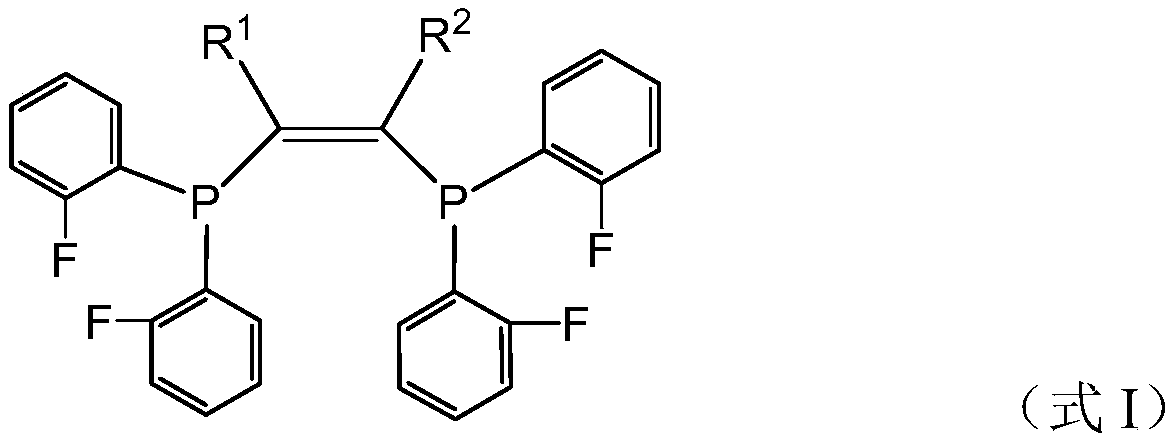
[0108] Preparation Example 1 is used to prepare fluorine-containing compound I 1 .
[0109]
[0110] Fluorochemical I 1 The preparation method refers to the above reaction formula, and the specific steps are as follows.
[0111] Under the protection of nitrogen, add 2-butyne (11mmol) and dry tetrahydrofuran 15mL in a 50mL reaction flask, then add n-butyllithium (11mmol) dropwise at 0°C (6.6mL n-butyllithium in hexane, n The concentration of butyllithium is 1.6M). After the dropwise addition, continue stirring at 0°C for 30 min, then add 2.2 g (10 mmol) of difluorophenylphosphine chloride dropwise, after the dropwise addition, raise the temperature to room temperature (25°C, the same below), and continue stirring for 2 h. Catalytic amounts of CuI and cesium carbonate were added, followed by 2.2 g (10 mmol) of difluorophenylphosphine chloride, the temperature was raised to 90° C. and stirred at 90° C. for 4 h. After the reaction, cool to room temperature, filter the react...
preparation example 2
[0115] Preparation example 2 is used to prepare fluorine-containing compound I 2 .
[0116] In this preparation example, the fluorine-containing compound was prepared by the same method as in preparation example 1, except that 2-butyne was replaced by 2,5-dimethyl-3-hexyne. The prepared compound is carried out nuclear magnetic resonance analysis, confirms that the prepared compound is the fluorine-containing compound shown in formula I, wherein, R 1 and R 2 for i Pr.
[0117] h 1 NMR (400MHz, CDCl 3 ): δ=7.35-7.00(m,16H), 2.70(m,2H), 1.15-1.10(m,12H).
preparation example 3
[0119] Preparation example 3 is used to prepare fluorine-containing compound I 3 .
[0120] In this preparation example, the fluorine-containing compound was prepared by the same method as in preparation example 1, except that 2-butyne was replaced by dicyclohexylacetylene. The prepared compound is carried out nuclear magnetic resonance analysis, confirms that the prepared compound is the fluorine-containing compound shown in formula I, wherein, R 1 and R 2 for Cy.
[0121] h 1 NMR (400MHz, CDCl 3 ): δ=7.35-6.99 (m, 16H), 2.15 (m, 2H), 1.30-1.60 (m, 20H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com