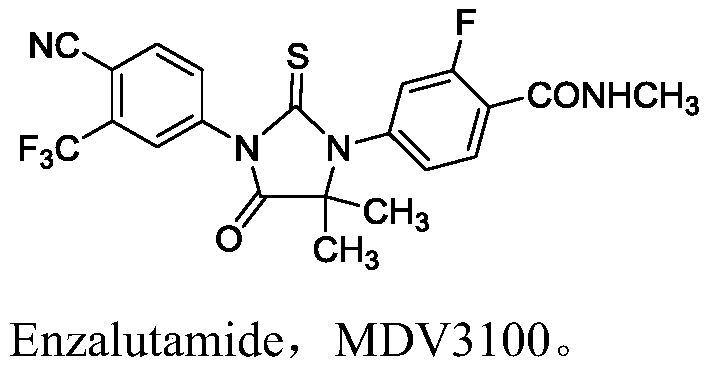
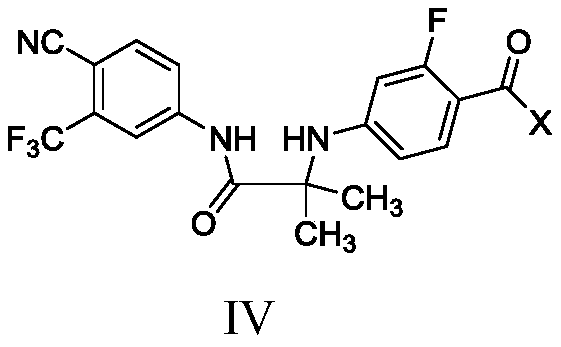
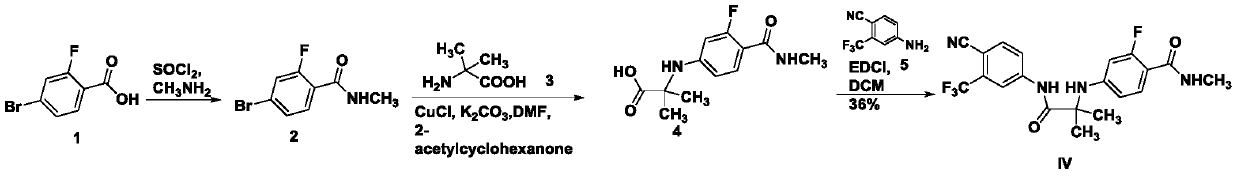
Method for preparation of compound shown as formula (IV) of enzalutamide synthesis intermediate
A technology of enzalutamide and compounds, which is applied in the field of chemical drug synthesis, can solve the problems of difficult removal of by-products and low yield of target products, and achieve the effects of simple post-treatment operation, high conversion rate and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Under the protection of nitrogen, add compound I (50g, 0.150mol), sodium iodide (67.5g, 0.450mol) and 500mL of acetone to a 1000mL three-necked flask at room temperature, magnetically stir, and heat to reflux, react for 16h, stop the reaction , Concentrated to an appropriate volume to precipitate a solid, filtered with suction, washed with water, and dried to obtain an off-white solid, dried to obtain 45.8 g of the compound of formula II, with a yield of 80.0%.
Embodiment 2
[0024] Under the protection of nitrogen, into a 100mL three-necked flask, add compound II (2.67g, 0.007mol), compound III (X=OCH 3 ) (1.39g, 0.008mol), potassium carbonate (2.85g, 0.021mol) and acetonitrile (30mL), magnetically stirred and heated to reflux, react for 16h, stop the reaction, cool to room temperature, concentrate to 10mL, add 30mL of water After suction filtration and drying, 2.22 g of off-white solid was obtained, with a yield of 75.0%. 1 H-NMR(400M,DMSO-d6): 10.576(s,1H),8.324-8.319(d,1H,J=2.0Hz),8.220-8.193(dd,1H,J=8.8Hz,2.0Hz), 8.082 -8.061(d,1H,J=8.4Hz),7.652-7.608(t,1H,J=8.8Hz),7.065(s,1H),6.396-6.369(dd,1H,J=8.8Hz,2.0Hz) ,6.293-6.252(m,1H),3.721(s,3H),1.529(s,6H)
Embodiment 3
[0026] Under the protection of nitrogen, into a 100mL three-necked flask, add compound II (2.67g, 0.007mol), compound III (X=OCH 3 ) (1.39g, 0.008mol), diisopropylethylamine (2.66g, 0.021mol) and acetonitrile (30mL), magnetically stirred, and heated to reflux, react overnight, stop the reaction, cool to room temperature, concentrate to 10mL, 30 mL of citric acid aqueous solution was added, suction filtered and dried to obtain a crude brown solid. Petroleum ether / ethyl acetate column chromatography was used to obtain 1.67 g of off-white solid, with a yield of 56.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com