Electrolyte for zinc anode alkaline secondary battery
A secondary battery, electrolyte technology, applied in the direction of alkaline storage battery, alkaline storage battery, nickel storage battery, etc. performance, improved stability, less precipitation or precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
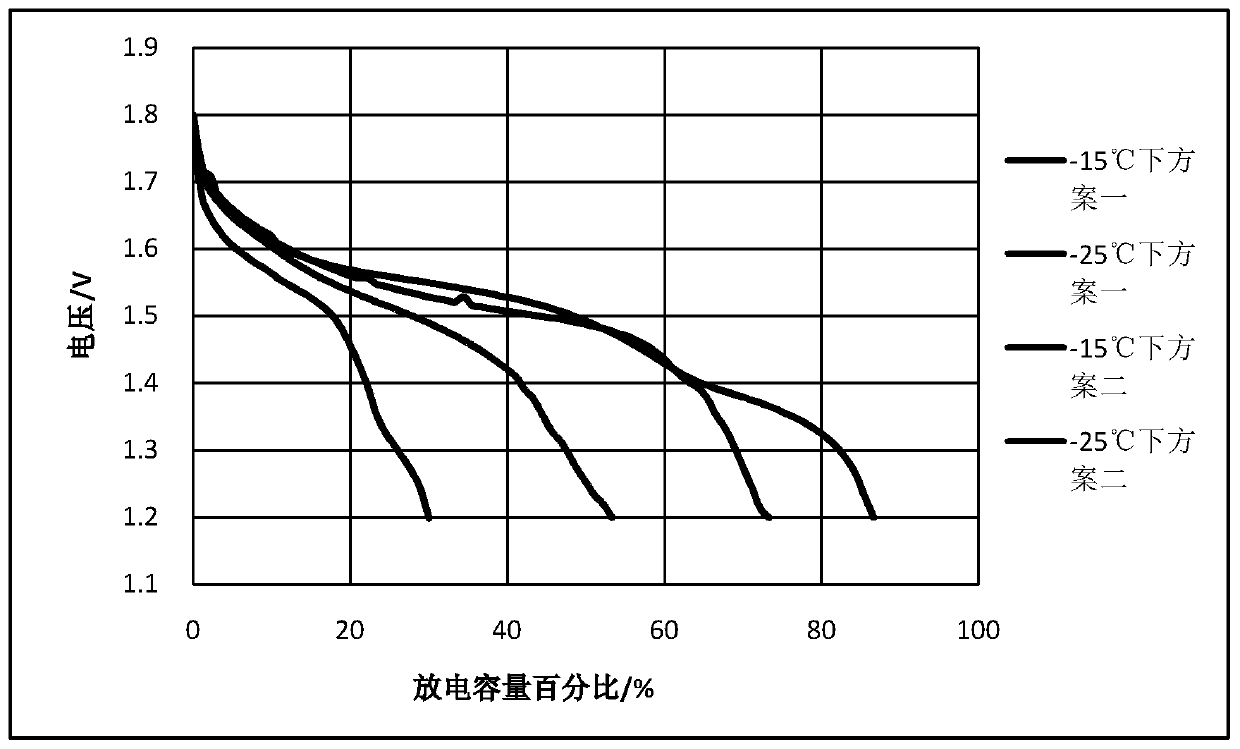
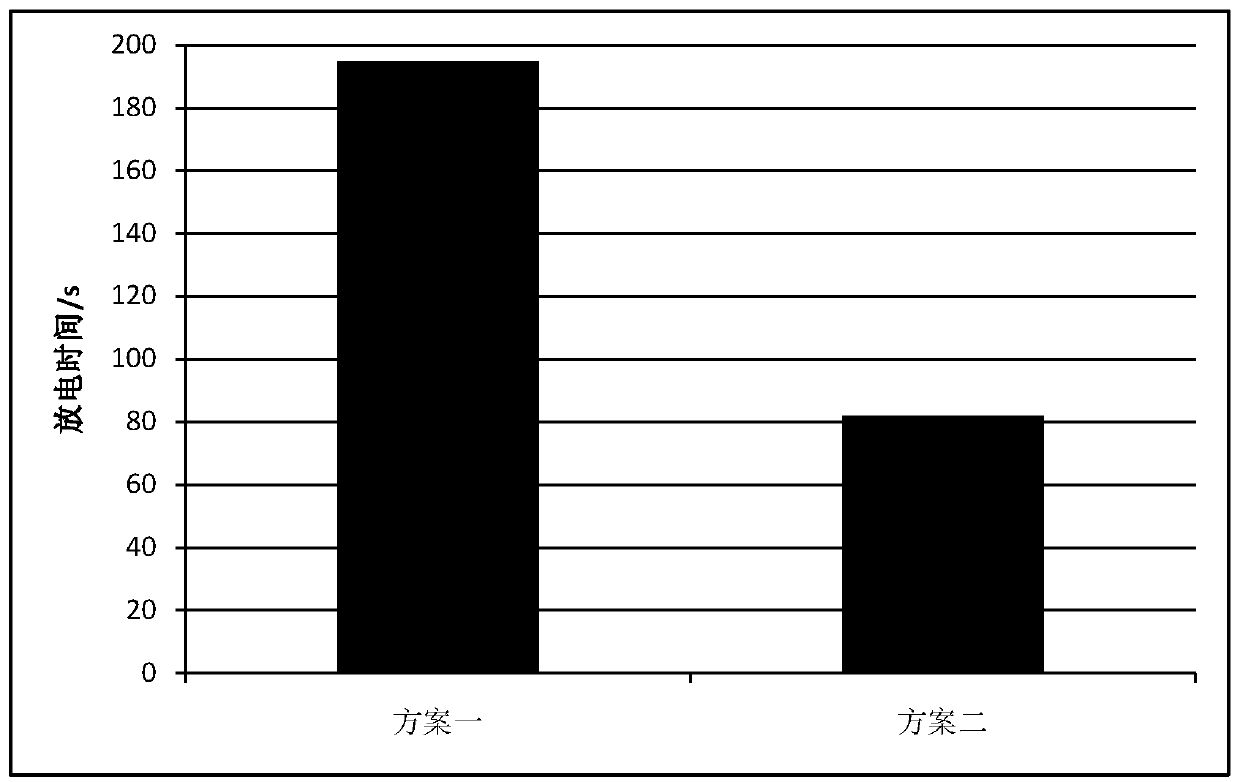
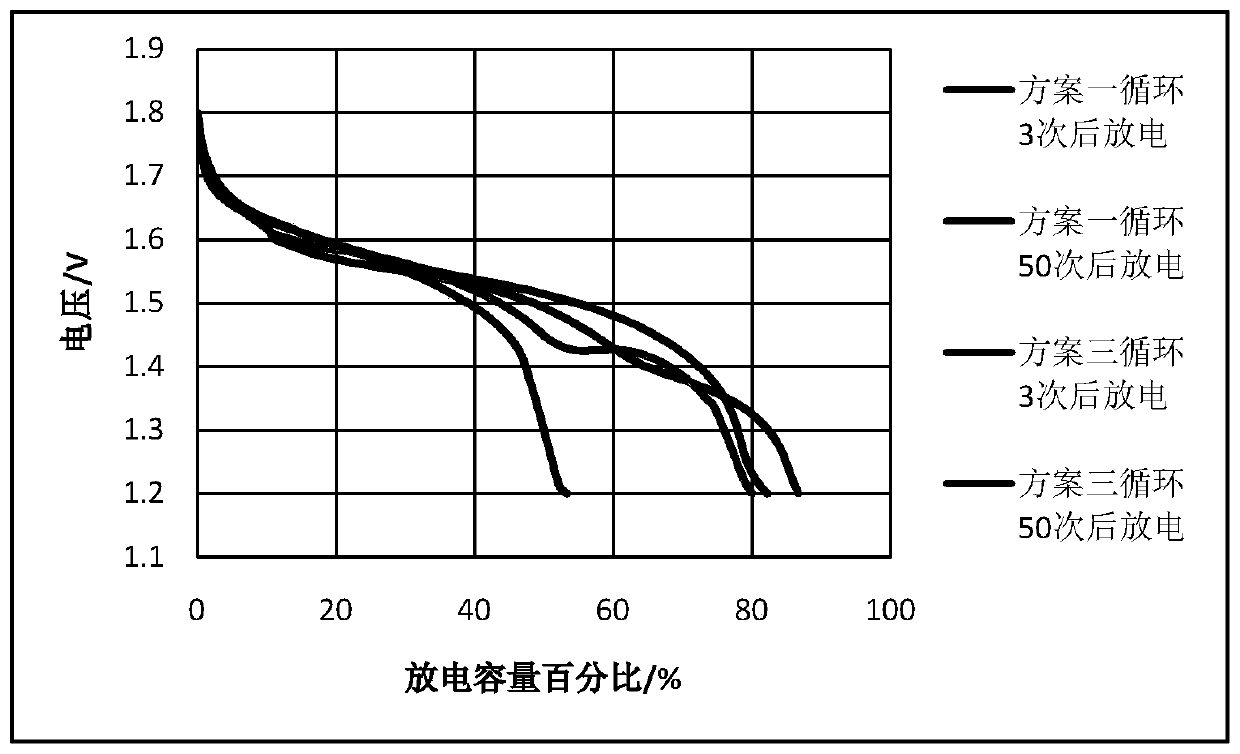
[0032] It is a 1.6V10Ah square zinc-nickel battery assembled with the electrolyte of all the above formulas; the electrolyte includes A, B, C, D, E, F; where A is KOH, B is NaOH, C is boric acid, and D It is fumed silica, E is zinc oxide, F is pure water; A is 10mol / L, B is 1mol / L, C is 0.3mol / L, D is 0.015mol / L, and E is 1.5mol / L. According to the concentration ranges of the above-mentioned solutes, add water, mix and stir evenly to obtain the electrolyte solution of this embodiment.
[0033] The electrolyte solution of this embodiment was assembled into a 1.6V10Ah square zinc-nickel battery for use.
[0034] S1. Mix and dissolve A (total amount of A) / n and part of pure water F to form the first conductive solution, wherein n=6;
[0035] S2. Mix and dissolve B and the first conductive solution to form a second conductive solution;
[0036] S3, mixing and dissolving C in the second conductive solution in an amount of c (total amount of C) / n, mixing and dissolving A of a / n af...
Embodiment 2
[0045] It is a 1.6V10Ah square zinc-nickel battery assembled with the electrolyte of all the above formulas; the electrolyte includes A, B, C, D, E, F; where A is KOH, B is LiOH, C is boric acid, and D It is fumed silica, E is zinc oxide, F is pure water; A is 12mol / L, B is 1.2mol / L, C is 0.3.6mol / L, D is 0.018mol / L, E is 1.8mol / L . According to the concentration ranges of the above-mentioned solutes, add water, mix and stir evenly to obtain the electrolyte solution of this embodiment.
[0046] The electrolyte solution of this embodiment was assembled into a 1.6V10Ah square zinc-nickel battery for use.
[0047] S1. Mix and dissolve A (total amount of A) / n and part of pure water F to form the first conductive solution, wherein n=8;
[0048] S2. Mix and dissolve B and the first conductive solution to form a second conductive solution;
[0049] S3, mixing and dissolving C in the second conductive solution in an amount of c (total amount of C) / n, mixing and dissolving A of a / n ...
Embodiment 3
[0055] Embodiment 3 (comparative example)
[0056] This embodiment is a 1.6V10Ah square zinc-nickel battery assembled from an electrolyte that does not contain components C and D;
[0057] The electrolyte includes A, B, E, and F; where A is KOH, B is NaOH, E is zinc oxide, and F is pure water; A is 10mol / L, B is 1mol / L, and E is 1.5mol / L. According to the concentration ranges of the above-mentioned solutes, add water, mix and stir evenly to obtain the electrolyte solution of this embodiment.
[0058] The electrolyte solution of this embodiment was assembled into a 1.6V10Ah square zinc-nickel battery for use. The preparation steps are the same as in Example 1 except that C and D are not added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com