Preparation method of electrolyte and zinc-nickel battery using electrolyte
A preparation method and electrolyte technology, which are applied in nickel storage batteries, alkaline storage batteries, composite electrolytes, etc., can solve the problems of reduced electrolyte performance, incomplete dissolution, and increased difficulty in the dissolution process, so as to suppress negative electrode dendrites and prevent The effect of anode passivation, reasonable and efficient energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
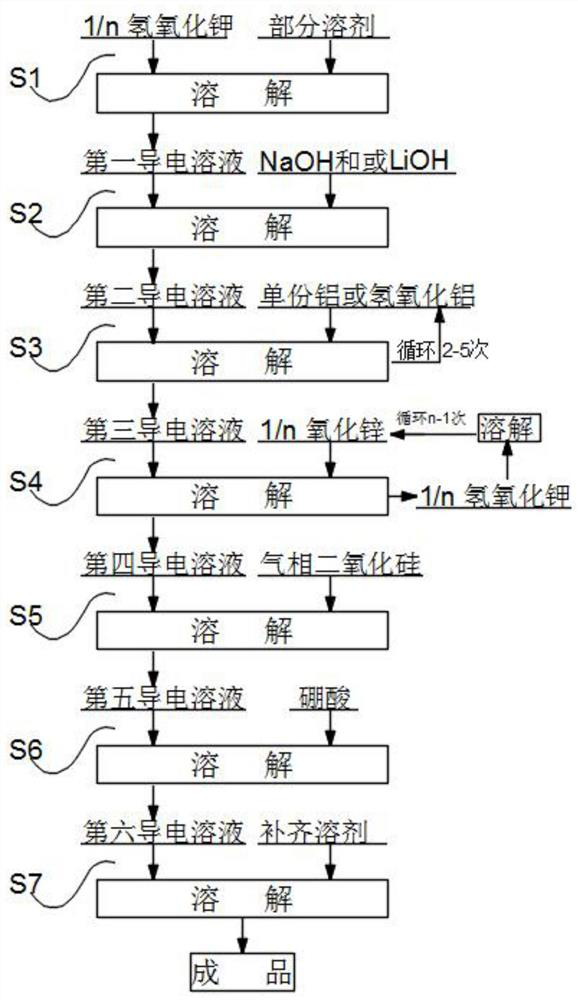
[0048]The composition of the electrolyte in this embodiment is as follows: select potassium hydroxide as 400g potassium hydroxide, 25g lithium hydroxide, 2g aluminum hydroxide, 82g zinc oxide, 0.17g fumed silica, 13.6 boric acid, 866g of pure water.
[0049]S1. Weigh 600 g of pure water in the reaction vessel, add 50 g of potassium hydroxide to the pure water, stir and heat, and obtain the first conductive solution after the potassium hydroxide is completely dissolved;
[0050]S2. Add 25 g of lithium hydroxide to the first conductive solution, keep stirring and heat, and wait until the lithium hydroxide is completely dissolved to obtain a second conductive solution;
[0051]S3. Add aluminum hydroxide to the second conductive solution twice, adding 1g of aluminum hydroxide each time. After the aluminum hydroxide added last time is completely dissolved, perform the next step of dissolving until the aluminum hydroxide is completely Add and dissolve to obtain a third conductive solution;
[0052]S4...
Embodiment 2
[0060]The composition of the electrolyte in this embodiment is as follows: the electrolyte potassium hydroxide is 536 g of potassium hydroxide, 45 g of a mixture of sodium hydroxide and lithium hydroxide, and the ratio of sodium hydroxide and lithium hydroxide is 2:1 by weight. , That is, 30g of sodium hydroxide, 15g of lithium hydroxide, 0.8g of aluminum, 122g of zinc oxide, 0.3 of fumed silica, 21.4g of boric acid, 782g of pure water.
[0061]S1. Weigh 650 g of pure water in the reaction vessel, add 134 g of potassium hydroxide to the pure water, stir and heat, and obtain the first conductive solution after the potassium hydroxide is completely dissolved;
[0062]S2. Add 45 g of a mixed solution of sodium hydroxide and lithium hydroxide to the first conductive solution, keep stirring and heating, and wait until the mixed solution of sodium hydroxide and lithium hydroxide is completely dissolved to obtain a second conductive solution;
[0063]S3. Add aluminum to the second conductive soluti...
Embodiment 3
[0071]The composition of the electrolyte in this embodiment is as follows: 400 g of potassium hydroxide, 25 g of lithium hydroxide, 2 g of aluminum hydroxide, 82 g of zinc oxide, 0.17 g of fumed silica, 13.6 g of boric acid, and 866 g of pure water.
[0072]An electrolyte preparation method, the preparation steps of the electrolyte are as follows:
[0073]S1. Weigh 600g of pure water in the reaction vessel, add 40g of potassium hydroxide, stir and heat, and mix and dissolve into the first conductive solution;
[0074]S2. Mix and dissolve 25g of lithium hydroxide and the first conductive solution into a second conductive solution, and keep stirring and heating;
[0075]S3. Dissolve 2g of alumina in the second conductive solution five times, keep stirring and heating, and finally prepare the third conductive solution;
[0076]S4. Mix and dissolve 8.2g zinc oxide in the third conductive solution. After 2 minutes, mix and dissolve 40g potassium hydroxide. Repeat 9 times to prepare the fourth conductiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com