Method for one-step acid leaching of laterite-nickel ore and co-production of lithium iron phosphate positive electrode active material
A cathode active material, laterite nickel ore technology, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of low leaching selectivity and unsatisfactory electrical performance of materials, achieve uniform particle and size, improve Electron conductivity and lithium ion diffusivity, and the effect of improving battery specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
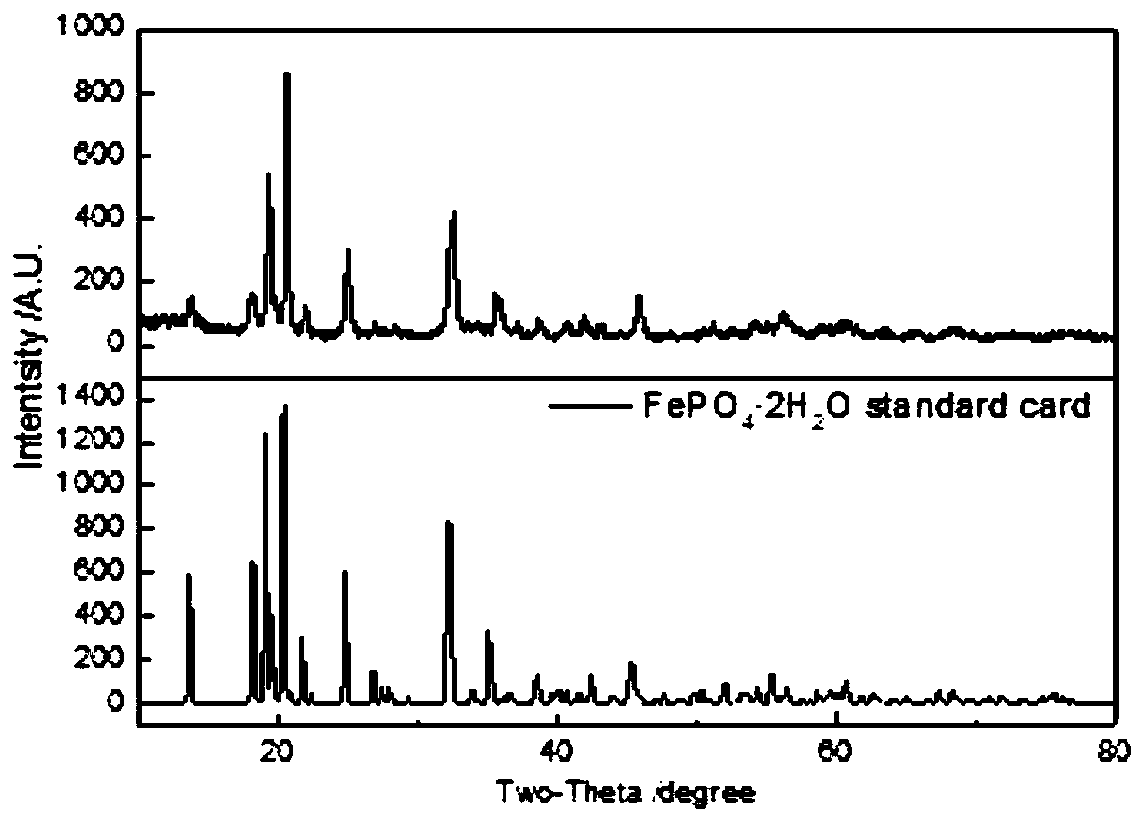
[0056] Crush the laterite nickel ore to obtain laterite nickel ore powder with a particle size of 1 mm, add phosphoric acid solution to the laterite nickel ore, and mix to obtain laterite nickel ore slurry; wherein, the ratio of the acid solution to the laterite nickel ore mass is 10:1; the phosphoric acid solution in the phosphoric acid solution The mass ratio of mass to laterite nickel ore is 3:1. The ore slurry is poured into a sealed reactor, and stirred and leached at a temperature of 130°C for 90 minutes, wherein the leaching pressure is 0.27MPa, and the stirring speed is 30rpm. The yield of iron phosphate is 1.69 times of the quality of laterite nickel ore, and the phase analysis of iron phosphate is as follows: figure 1 , the main component is ferric phosphate dihydrate; the main components of ferric phosphate are as shown in Table 2, with a purity of 98%, and doped with metals such as aluminum and manganese, wherein the iron / phosphorus molar ratio is 0.9, (iron+alumin...
Embodiment 2
[0061] Crush the laterite nickel ore to obtain laterite nickel ore powder with a particle size of 1 mm, add phosphoric acid solution to the laterite nickel ore, and mix to obtain laterite nickel ore slurry; wherein, the ratio of the acid solution to the laterite nickel ore mass is 6:1; the phosphoric acid solution in the phosphoric acid solution The mass ratio of mass to laterite nickel ore is 3:1. The ore slurry is poured into a sealed reactor, and stirred and leached at a temperature of 120°C for 90 minutes, wherein the leaching pressure is 0.2MPa, and the stirring speed is 30rpm. The yield of iron phosphate is 1.65 times of the quality of laterite nickel ore, and the main component is iron phosphate dihydrate with a purity of 98%. The result is similar to that of Example 1.
[0062] After drying the obtained iron phosphate product, it is evenly mixed with lithium carbonate at a molar ratio of 1:1 and then roasted to obtain a lithium iron phosphate positive electrode materia...
Embodiment 3
[0064] Crush the laterite nickel ore to obtain laterite nickel ore powder with a particle size of 1mm, add phosphoric acid solution to the laterite nickel ore, and mix to obtain laterite nickel ore slurry; wherein, the ratio of the acid solution to the laterite nickel ore mass is 8:1; the phosphoric acid solution in the phosphoric acid solution The mass ratio of mass to laterite nickel ore is 4:1. The ore slurry is poured into a sealed reaction kettle, and stirred and leached at a temperature of 140°C for 60 minutes, wherein the leaching pressure is 0.36MPa, and the stirring speed is 30rpm. The yield of iron phosphate is 1.70 times of the mass of laterite nickel ore. The main component is ferric phosphate dihydrate with a purity of 98%, and the result is similar to Example 1.
[0065] After drying the obtained iron phosphate product, it is evenly mixed with lithium carbonate at a molar ratio of 1:1 and then roasted to obtain a lithium iron phosphate positive electrode materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com