immune combination for inducing broad-spectrum neutralizing antibodies against HIV-1
A technology of HIV-1 and antibody, applied in the field of vaccines, to achieve the effect of prolonging the existence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
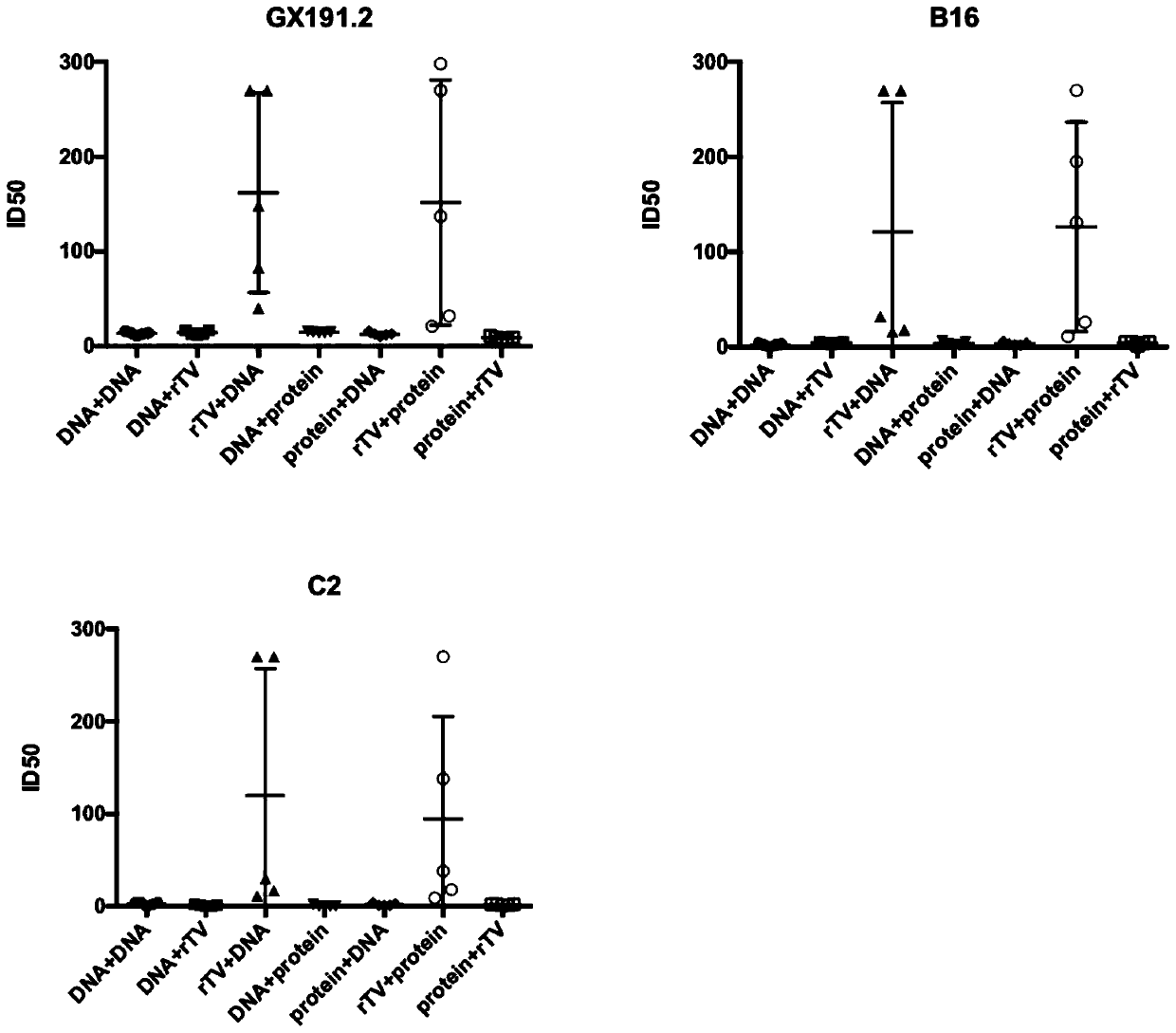
[0110] Example 1: Evaluation of the Induction Effect of the Second Injection of Different Immunization Combinations on Broadly Neutralizing Antibodies in Mice
[0111] The mice were immunized with different immunization combinations, and the titers of neutralizing antibodies against HIV-1 pseudoviruses GX191.2, B16 and C2 induced by different immunization combinations were evaluated after 4 weeks of immunization.
[0112] The experimental steps are as follows: the mice were randomly divided into 7 groups, which were named DNA+DNA group, rTV+rTV group, rTV+DNA group, DNA+protein group, protein+DNA group, rTV group respectively according to different immunogen combination forms. +protein group and protein+rTV group. The specific immunization combinations are shown in Table 2.
[0113] The titers of neutralizing antibodies against HIV-1 pseudoviruses GX191.2, B16 and C2 produced by different immunization combinations are as follows: figure 1 Shown: When the combination of the s...
Embodiment 2
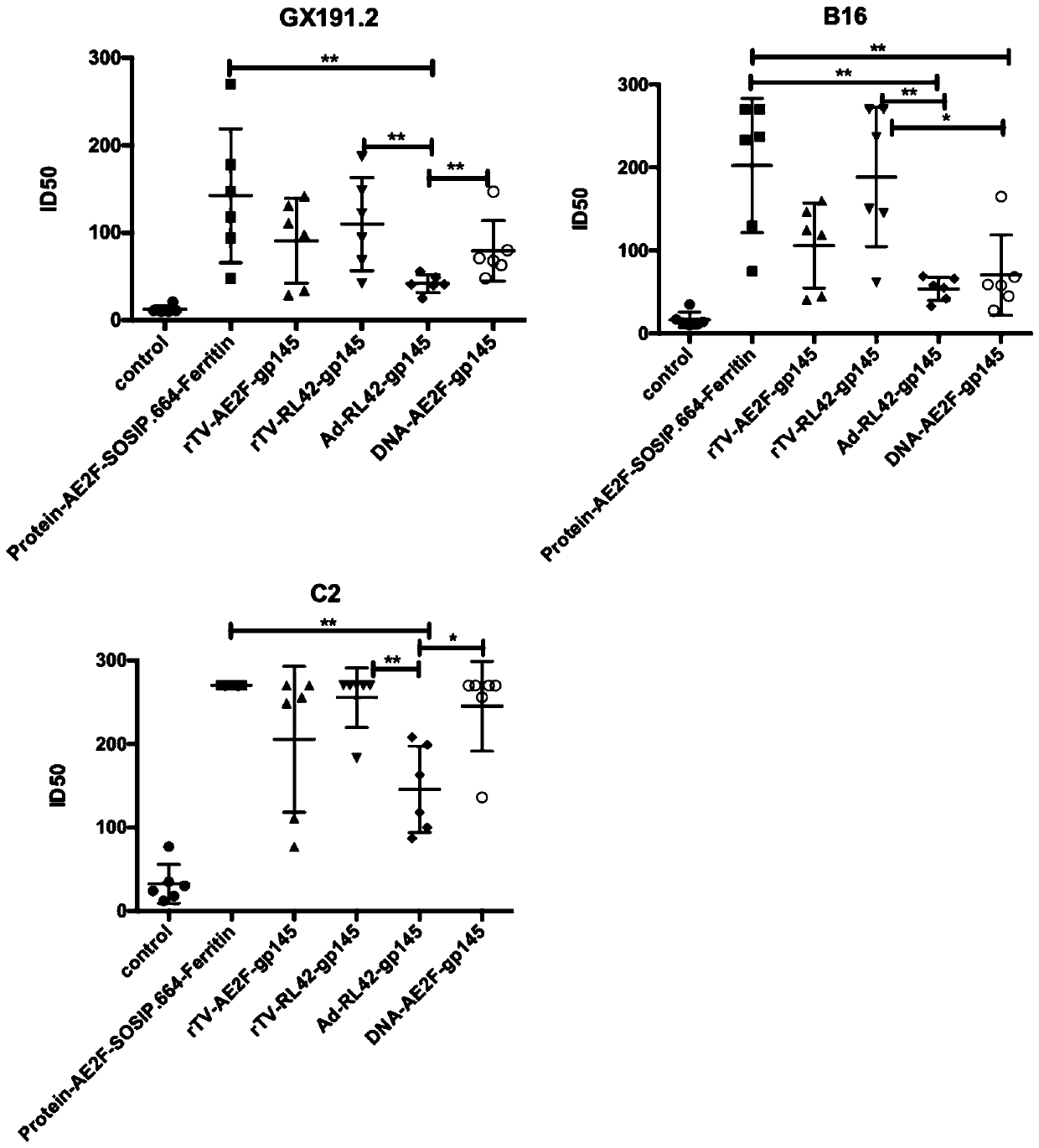
[0117] Example 2: Evaluation of the Inducing Effect of Different Immunization Combinations for the Third Injection on Widely Awareness Neutralizing Antibodies in Mice
[0118] According to the conclusion drawn in Example 1, the second dose is fixed as a poxvirus vector vaccine, and the third dose is optimized. C57 / BL6 mice were immunized with different immunization combinations. The immunogen was HIV-1 membrane protein, and the adjuvant was aluminum adjuvant. Titers of neutralizing antibodies to B16 and C2.
[0119] The experimental steps are as follows: the mice were randomly divided into 7 groups, which were named control group, protein-AE2F-SOSIP.664-ferritin group, rTV-AE2F-gp145 group, rTV-RL42-gp145 group according to the immunogen of the third injection group, Ad-RL42-gp145 group and DNA-AE2F-gp145 group. The specific immunization combinations are shown in Table 3. The titers of neutralizing antibodies against HIV-1 pseudoviruses GX191.2, B16 and C2 produced by diffe...
Embodiment 3
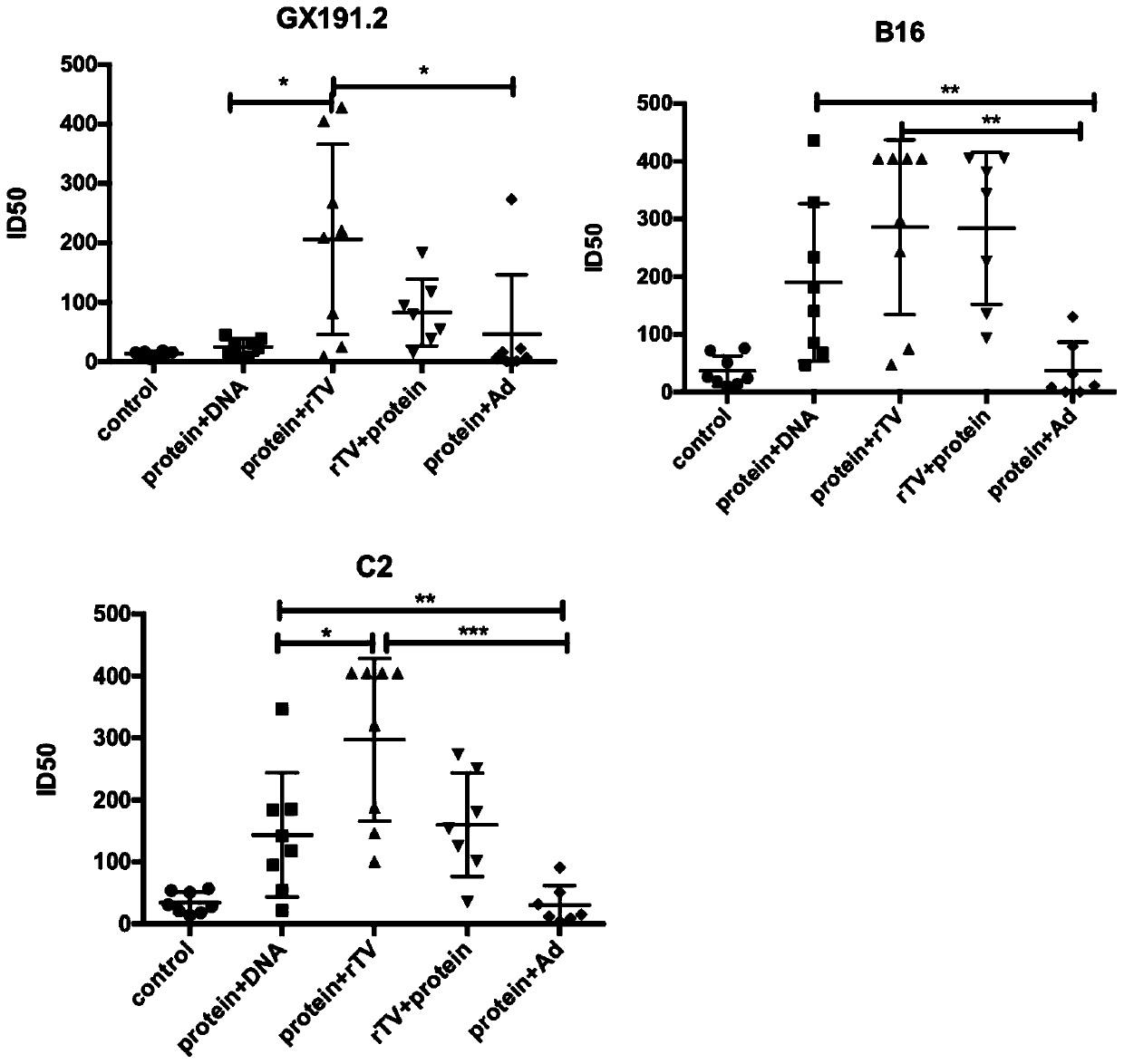
[0123] Example 3: Evaluation of the induction effect of broad-spectrum neutralizing antibodies in mice with different immunization combinations of the third and fourth doses
[0124] According to the conclusions drawn in Examples 1 and 2, the second dose was fixed as a poxvirus vector vaccine, and the recombinant protein vaccine and poxvirus vector vaccine that performed well in the third dose were selected, combined in three or four doses, and mice were immunized , 4 weeks after completion of immunization, evaluate the neutralizing antibody titers induced by different immunization combinations against HIV-1 pseudoviruses GX191.2, B16 and C2.
[0125] The experimental steps are as follows: the mice were randomly divided into 5 groups, which were named control group, protein+DNA group, protein+rTV group, rTV+protein group and protein+ Ad group. The specific immunization combinations are shown in Table 4.
[0126] The titers of neutralizing antibodies against HIV-1 pseudovirus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com