Diagnostic kit for combined diagnosis of mycoplasma pneumonia and application of diagnostic kit
A technology for mycoplasma pneumonia and diagnostic kits, which is applied in biological testing, material testing products, measuring devices, etc., can solve the problems of low sensitivity, poor specificity and high detection cost of the colloidal gold method, and achieve a short detection window period, simple operation, Strong specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
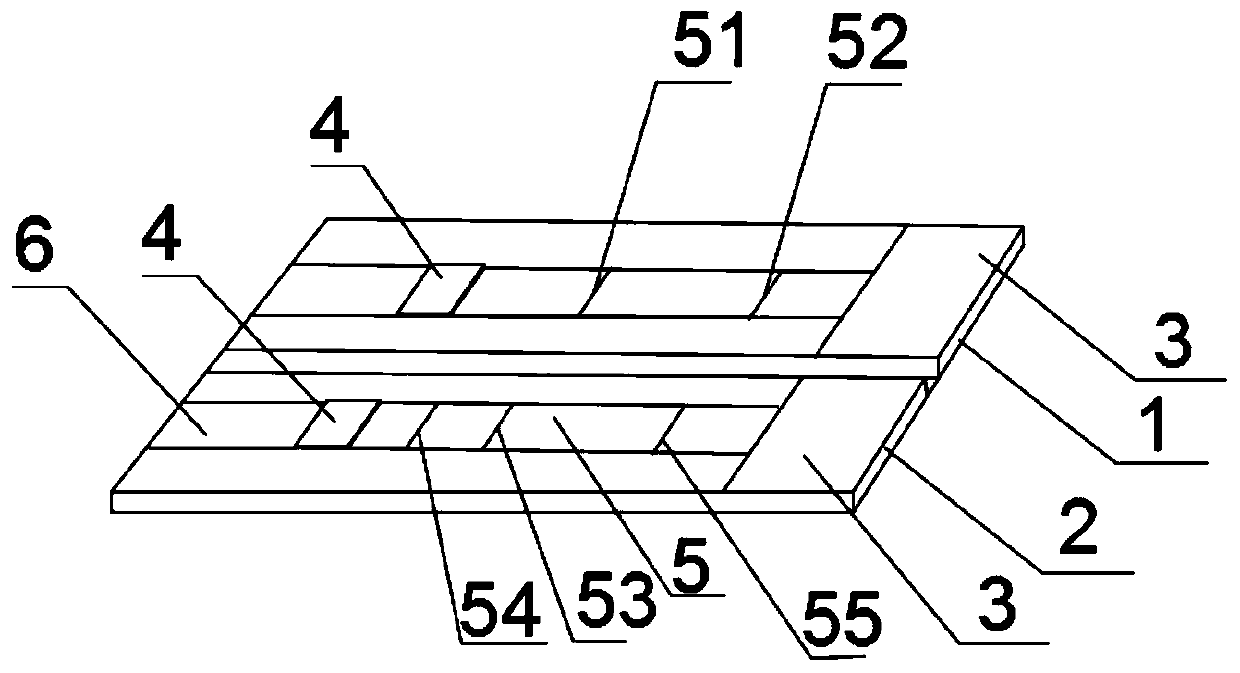
[0039] like figure 1 As shown, the present embodiment provides a diagnostic kit for the combined diagnosis of mycoplasma pneumonia, which includes a single unit that is provided with a bottom plate and sequentially connected sample pad 6, binding pad 4, reaction membrane 5 and water-absorbent pad 3 arranged on the bottom plate. Side test test strip and double test test strip 2;
[0040] A time-resolved fluorescent microsphere-labeled Mycoplasma pneumoniae antibody is immobilized on the binding pad 4 of the single test test strip 1,
[0041] The reaction membrane 5 of the single test test strip 1 is provided with a first detection line 51 and a first quality control line 52, the first detection line 51 is coated with Mycoplasma pneumoniae antibody, and the first quality control line 52 is coated with sheep antibody Mouse IgG antibody;
[0042] Time-resolved fluorescent microspheres labeled mouse anti-human IgM monoclonal antibody and mouse anti-human IgG antibody are immobili...
Embodiment approach 2
[0056] This embodiment also provides the application of the above-mentioned detection kit for the joint detection of mycoplasma pneumoniae in the joint detection of mycoplasma pneumoniae and its IgM / IgG. The sample is first added to the sample pad at 6 places, and then the diluent is added, and the reaction film is left to observe Color reaction on 5.
[0057] Further, the diluent is one or two of phosphate, Tris, hepes, boric acid, surfactant Tween 20, inert protein and sodium chloride and a combination thereof.
[0058] The sample diluent contains the following components in mass concentrations: 0.02M phosphate buffer, 0.02-0.05% surfactant Tween 20, 0.2-0.5% inert protein BSA and 0.85% sodium chloride.
Embodiment 1
[0061] The following kits were prepared:
[0062] Obtain the single-side detection test strip and the double-test detection test strip 2 of the sample pad 6, the binding pad 4, the reaction film 5 and the water-absorbing pad 3 that are all provided with the base plate and the sequentially connected sample pads arranged on the base plate; The binding pad 4 of strip 1 is immobilized with time-resolved fluorescent microsphere-labeled Mycoplasma pneumoniae antibody,
[0063] The reaction membrane 5 of the single test test strip 1 is provided with a first detection line 51 and a first quality control line 52, the first detection line 51 is coated with Mycoplasma pneumoniae antibody, and the first quality control line 52 is coated with sheep antibody Mouse IgG antibody;
[0064] Time-resolved fluorescent microspheres labeled mouse anti-human IgM monoclonal antibody and mouse anti-human IgG antibody are immobilized on the binding pad 4 of the double test test strip 2,
[0065] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com