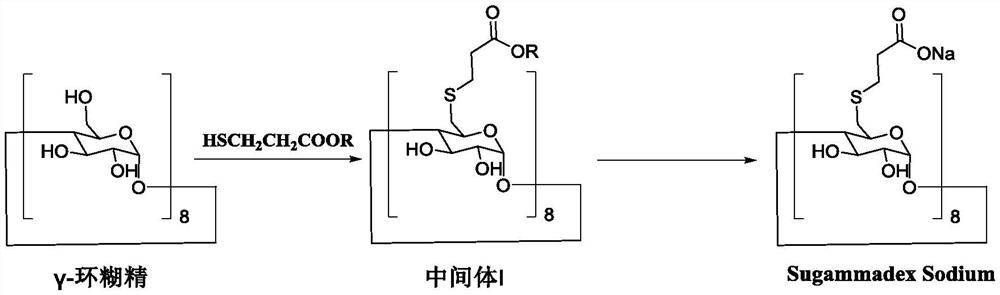
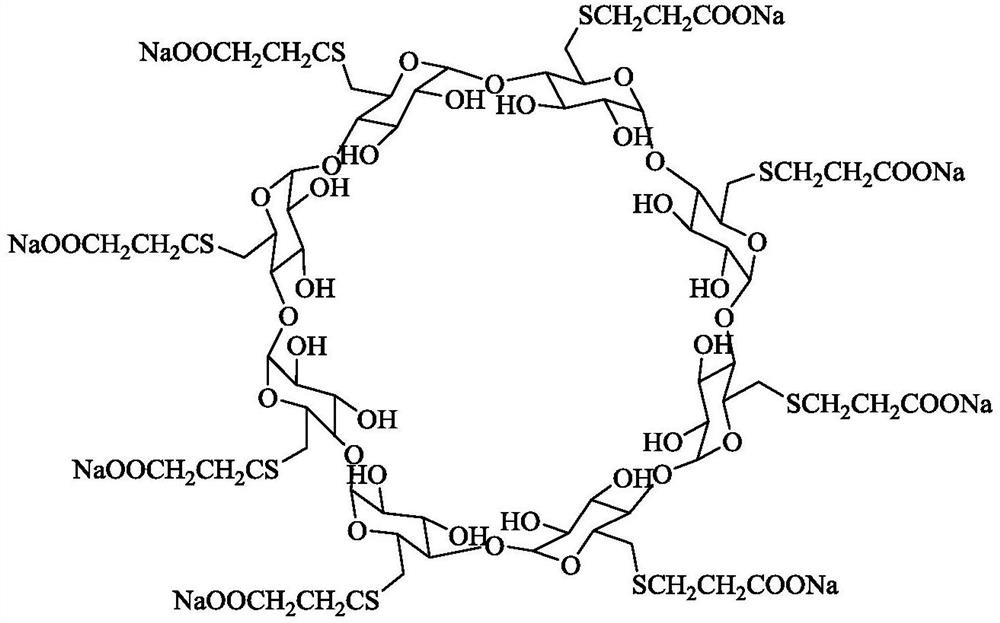
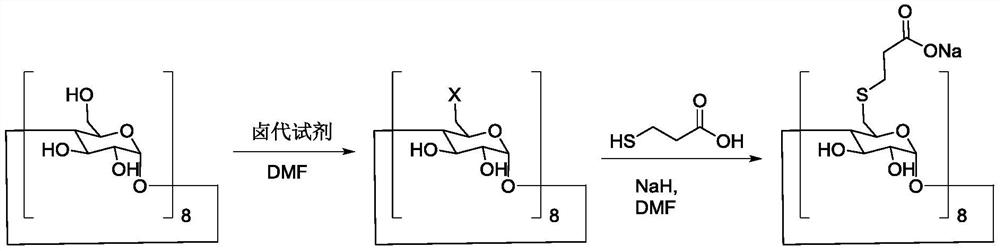
A kind of preparation method of sugammadex sodium
A technology of sugammadex sodium and reagents, which is applied in the field of medicinal chemistry, can solve the problems of many impurities and low conversion rate, and achieve the effects of high purity, simple and safe reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis of sugammadex ethyl ester (intermediate I)
[0076] Under argon protection and shading conditions, dry γ-cyclodextrin (64.86g, 0.05mol), ethyl 3-mercaptopropionate (75.15g, 0.56mol), triphenylphosphine (230.82g, 0.88mol) were mixed Add it to anhydrous dimethyl sulfoxide (1000mL), after all the materials are dissolved, control the temperature to 0~5℃ and add diisopropyl azodicarboxylate (DIAD, 177.94g, 0.88mol) dropwise. The temperature is 30~35 ℃ and react for 8 hours, the detection reaction is completed, the reaction is finished, filtered, methanol / purified water (V:V=2:1, 2000mL) is added to the filtrate for crystallization, after the crystallization is completed, the filter cake is 35~40 After vacuum drying at °C, ethyl sugammadex (intermediate I) was obtained with a yield of 96% and a purity of 98.52%.
Embodiment 2
[0078] Synthesis of sugammadex ethyl ester (intermediate I)
[0079] Under argon protection and shading conditions, dry γ-cyclodextrin (64.86g, 0.05mol), ethyl 3-mercaptopropionate (64.41g, 0.48mol), triphenylphosphine (230.82g, 0.88mol) were mixed Add it into anhydrous dimethyl sulfoxide (1000mL), after all the materials are dissolved, add diethyl azodicarboxylate (DEAD, 153.14g, 0.88mol) dropwise at a temperature of 0 to 5°C. After the addition is complete, the temperature is controlled. 30~35 ℃ of reaction for 8 hours, detection reaction is completed, end the reaction, filter, add methanol / purified water (V:V=2:1, 2000mL) to the filtrate for crystallization, filter after the crystallization, filter cake 35~40 ℃ After drying in vacuo, ethyl sugammadex (intermediate I) was obtained with a yield of 91% and a purity of 96.34%.
Embodiment 3
[0081] Synthesis of sugammadex ethyl ester (intermediate I)
[0082] Under argon protection and shading conditions, dry γ-cyclodextrin (64.86g, 0.05mol), ethyl 3-mercaptopropionate (60.38g, 0.45mol), triphenylphosphine (230.82g, 0.88mol) Add it into anhydrous dimethyl sulfoxide (1000mL), after all the materials are dissolved, add diethyl azodicarboxylate (DEAD, 153.12g, 0.88mol) dropwise at a temperature of 0 to 5°C, after the dropwise addition is completed, the temperature is controlled 30~35 ℃ of reaction for 8 hours, detection reaction is completed, end the reaction, filter, add methanol / purified water (V:V=2:1, 2000mL) to the filtrate for crystallization, filter after the crystallization, filter cake 35~40 ℃ After vacuum drying, ethyl sugammadex (intermediate I) was obtained with a yield of 85% and a purity of 91.80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com