Oncolytic virus (oncolytic immunotherapy) capable of effectively treating even metastatic cancer while ensuring safety, with expression control system providing optimal expression level of mounted immunogenic gene
A technology of tumor dissolution and immune induction, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, viruses, etc., can solve difficult, difficult gene recombination, efficient and standardized production of conditional replication adenovirus Technology does not exist and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
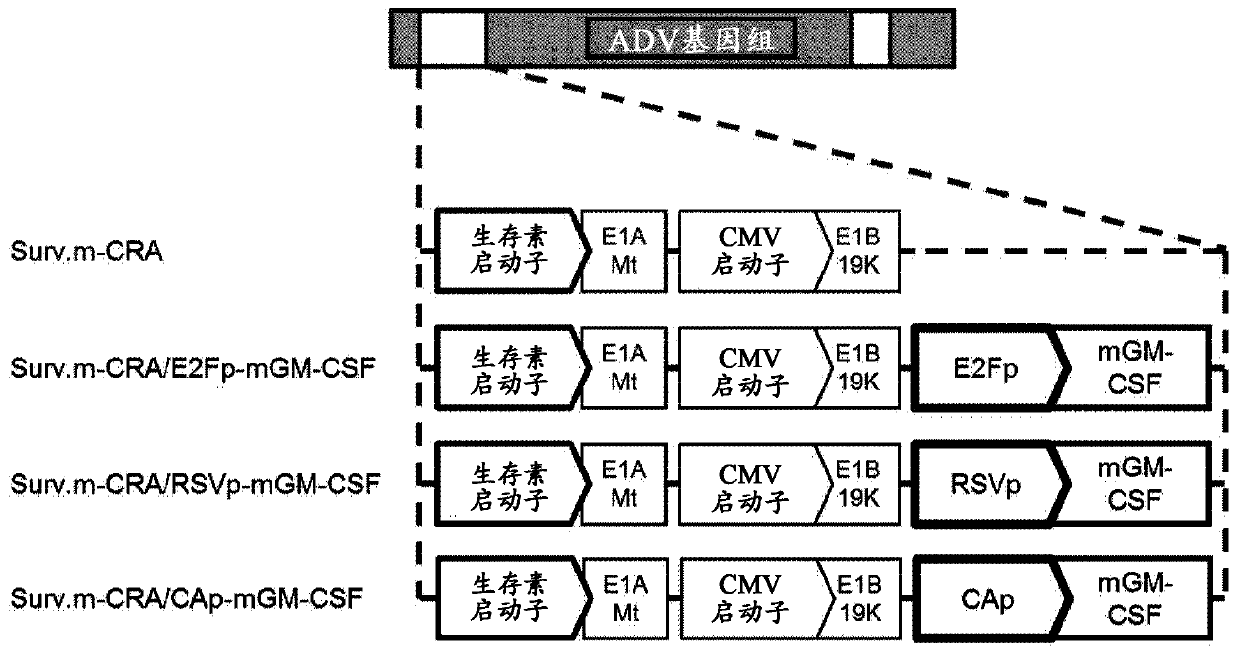
[0115] (Example 1) GM-CSF expresses the structure of Surv.m-CRA
[0116] The Surv.m-CRA with the survivin promoter integrated upstream of the early gene E1A necessary for adenovirus growth was inserted. The promoters of the E2F promoter, RSV promoter, and CA promoter were linked to the mouse GM-CSF cDNA. And the expression box that obtains, thereby constructs following three kinds of GM-CSF expression Surv.m-CRA ( figure 1 ). The construction of adenovirus was carried out based on the method described in GeneTherapy (2005, 12:1385-1393) by Nagano et al.
[0117] (1) Production of P1+3 plasmid
[0118] As the first stage of production, P1+3 was produced from the growth control plasmid P1 and the adenovirus backbone plasmid P3 (pAd.HM4, pAd.HM10; Mizuguchi and Kay, Hum. Gene Ther. 1999) by restriction enzyme treatment.
[0119] The adenovirus backbone plasmid P3 is loaded with human adenovirus type 5 genomic DNA, but lacks the E1 gene region required for virus propagation. I...
Embodiment 2
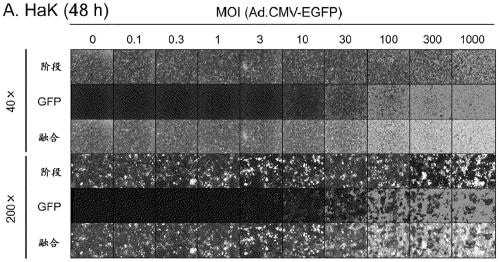
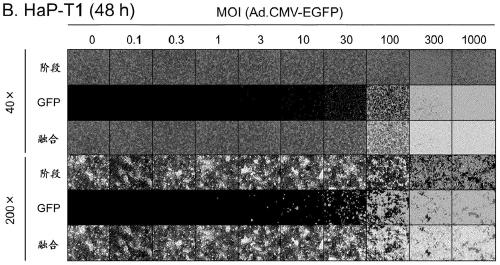
[0148] (Example 2) Infection efficiency of adenovirus in hamster-derived cells
[0149] The infection efficiency of adenovirus in hamster-derived cancer cells (HaK and HaP-T1) and normal cells (BHK-21) was examined.
[0150] HaK cells derived from Syrian hamster kidney tumor were provided by Prof. M. Wold, Saint Louis University School of Medicine. Use DMEM (Dulbecco's Modified Eagle Medium; Nacalai Tesque) containing 10% fetal bovine serum (FBS; Biowest) and 1% penicillin-streptomycin (Nacalai Tesque) at 37° C. , 5%CO 2 cultured under conditions. HaP-T1 cells derived from Syrian hamster pancreatic tumor were purchased from RIKEN BRC Cell Bank (RBRC-RCB0411). With 10% FBS, 1% non-essential amino acids (NEAA; SIGMA), 1 mM sodium pyruvate (Thermo Fisher Scientific) and 1% penicillin-streptomycin MEM (Minimum Essential Medium; SIGMA) at 37°C, 5% CO 2cultured under conditions. BHK-21 cells derived from Syrian suckling hamster kidney were purchased from JCRB Cell Bank (JCRB90...
Embodiment 3
[0153] (Example 3) Promoter activity in hamster-derived cells
[0154] The promoter activities of E2F, RSV, and survivin in hamster-derived cancer cells (HaK and HaP-T1) and normal cells (BHK-21) were investigated. On the day of infection, the cells seeded in the 6-well plate the day before were counted, and the result was: HaK was 1.29×10 6 cells / well, HaP-T1 is 1.0×10 6 cells / well, BHK-21 is 1.7×10 6 cells / well. Next, Ad.dE1.3 (control) in which genes E1 and E3 important for virus growth have been deleted, and Ad.E2Fp expressing the β-galactosidase gene (LacZ) under the control of each promoter -LacZ, Ad.RSVp-LacZ and Ad.Survp-LacZ were infected for 1 hour under the condition of MOI30, and cultured for 48 hours. Then, the β-galactosidase activity in the cell lysate was examined using the Beta-Glo (registered trademark) Assay System (Promega). Experiments were performed in groups of three wells under each condition, and the data were expressed as mean ± standard error. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com