Cyclobenzaprine sustained-release granule
A technology of cyclobenzaprine and sustained-release granules, which is applied in the field of medicine, can solve the problems of slow elimination, poor patient compliance, influence on curative effect, etc., and achieve the effects of reducing dosage, convenient taking and stable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
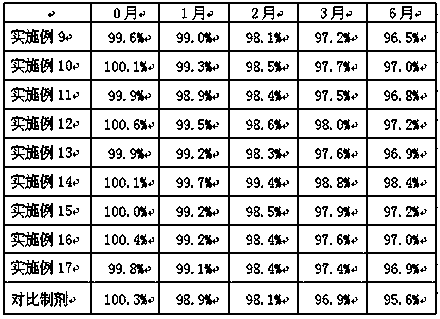
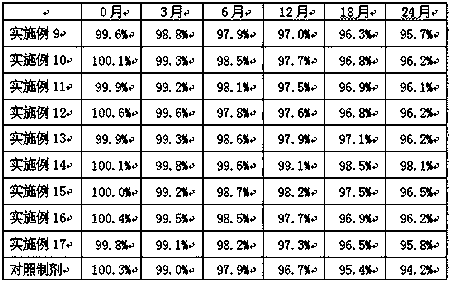
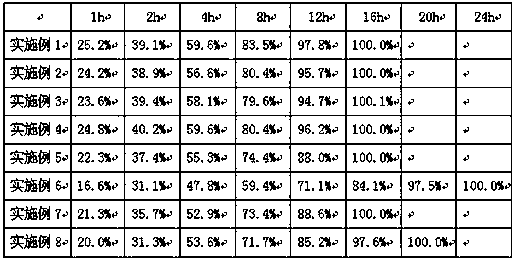
Examples
preparation example Construction
[0026] A preparation method for cyclobenzaprine sustained-release granules, comprising the following steps:
[0027] (1) Mix cyclobenzaprine and filler evenly, and use 85% ethanol as a binding agent to make a 20-24 purpose quick-release granule core for subsequent use;
[0028] (2) Dissolving the slow-release material, plasticizer, porogen, and anti-tack agent with 80% ethanol to make a slow-release coating solution;
[0029] (3) Evenly spray the prepared sustained-release coating solution on the surface of the immediate-release granule core prepared in step (1), and obtain cyclobenzaprine sustained-release granules after drying.
Embodiment 1
[0030] Example 1 Preparation of Cyclobenzaprine Sustained Release Granules
[0031] Cyclobenzaprine sustained-release granules are prepared according to the raw and auxiliary materials in the following prescription and the above-mentioned preparation method.
[0032] Cyclobenzaprine 25g
[0033] Microcrystalline Cellulose 325g
[0034] Carboxymethyl ethyl cellulose 200g
[0035] Dibutyl phthalate 100g
[0036] Povidone 50g
Embodiment 2
[0038] Example 2 Preparation of cyclobenzaprine sustained-release granules
[0039] Cyclobenzaprine sustained-release granules are prepared according to the raw and auxiliary materials in the following prescription and the above-mentioned preparation method.
[0040] Cyclobenzaprine 25g
[0041] Microcrystalline Cellulose 325g
[0042] Carboxymethyl ethyl cellulose 200g
[0043] Dibutyl phthalate 100g
[0044] Sucrose 50g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com