Clonidine hydrochloride sustained-release capsule
A technology of clonidine hydrochloride and sustained-release capsules, which is applied in the field of medicine, can solve the problems of the introduction of sustained-release effect, unknown indicators such as dissolution rate, and uneven content, and achieve uniform release, good patient compliance, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A preparation method of clonidine hydrochloride sustained-release capsules, comprising the following steps:
[0021] (1) Take a certain amount of starch and put it in a centrifugal granulator, use water as a binder, and make a blank pellet core with a particle size of 40-60 mesh;
[0022] (2) take clonidine hydrochloride, filler and binder by weight percentage, binder is made into 2~6% aqueous solution, clonidine hydrochloride and filler are put in the powder supply chamber of centrifugal granulator, Take an appropriate amount of the blank pellet core prepared in step (1) and put it in a granulation pot, use the aqueous solution of the binder as the binder to prepare drug-containing pellets, dry the pellets at 50-60°C after they are taken out of the pot, and sieve for 15 ~25 mesh for the next step of coating;
[0023] (3) Dissolve the slow-release material, plasticizer, porogen, and anti-tack agent with 65-85% ethanol solution to make a slow-release coating solution; ...
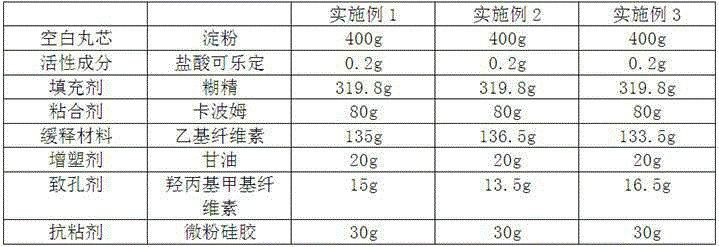
Embodiment 1~3
[0027] The preparation of embodiment 1~3 clonidine hydrochloride sustained release capsule
[0028] According to the raw and auxiliary materials in the table below, according to the above preparation method, taking the 0.1mg specification as an example, and using the ratio of 40% for the blank core, 40% for the drug-containing layer, and 20% for the coating layer, each example was prepared respectively 2000 clonidine hydrochloride extended-release capsules. The weight ratio of the slow-release material to the porogen in Example 1 is 9:1, the weight ratio of the slow-release material to the porogen in Example 2 is 10:1, and the weight ratio of the slow-release material to the porogen in Example 3 is The ratio is 8:1.
[0029]
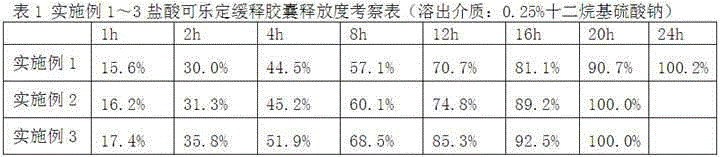
[0030] Test Example 1 Determination of Release of Clonidine Hydrochloride Sustained-release Capsules Gained in Examples 1-3
[0031] According to the "Guidelines for Sustained-release, Controlled-release and Delayed-release Preparations" in the 2015...
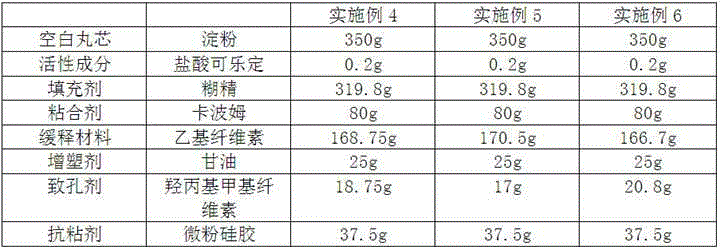
Embodiment 4~6
[0034] The preparation of embodiment 4~6 clonidine hydrochloride sustained-release capsules
[0035] According to the raw and auxiliary materials in the following table, according to the above-mentioned preparation method, taking 0.1 mg specification as an example, 2000 clonidine hydrochloride sustained-release capsules were prepared in each embodiment. According to the ratio of 35% for the blank core, 40% for the drug-containing layer, and 25% for the coating layer, 2000 clonidine hydrochloride sustained-release capsules were respectively prepared in each embodiment. The weight ratio of the slow-release material and the porogen in Example 4 is 9:1, the weight ratio of the slow-release material in Example 5 and the porogen is 10:1, and the weight of the slow-release material in Example 6 and the porogen The ratio is 8:1.
[0036]
[0037] Test Example 2 Determination of Release of Clonidine Hydrochloride Sustained-release Capsules Gained in Examples 4-6
[0038] The measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com