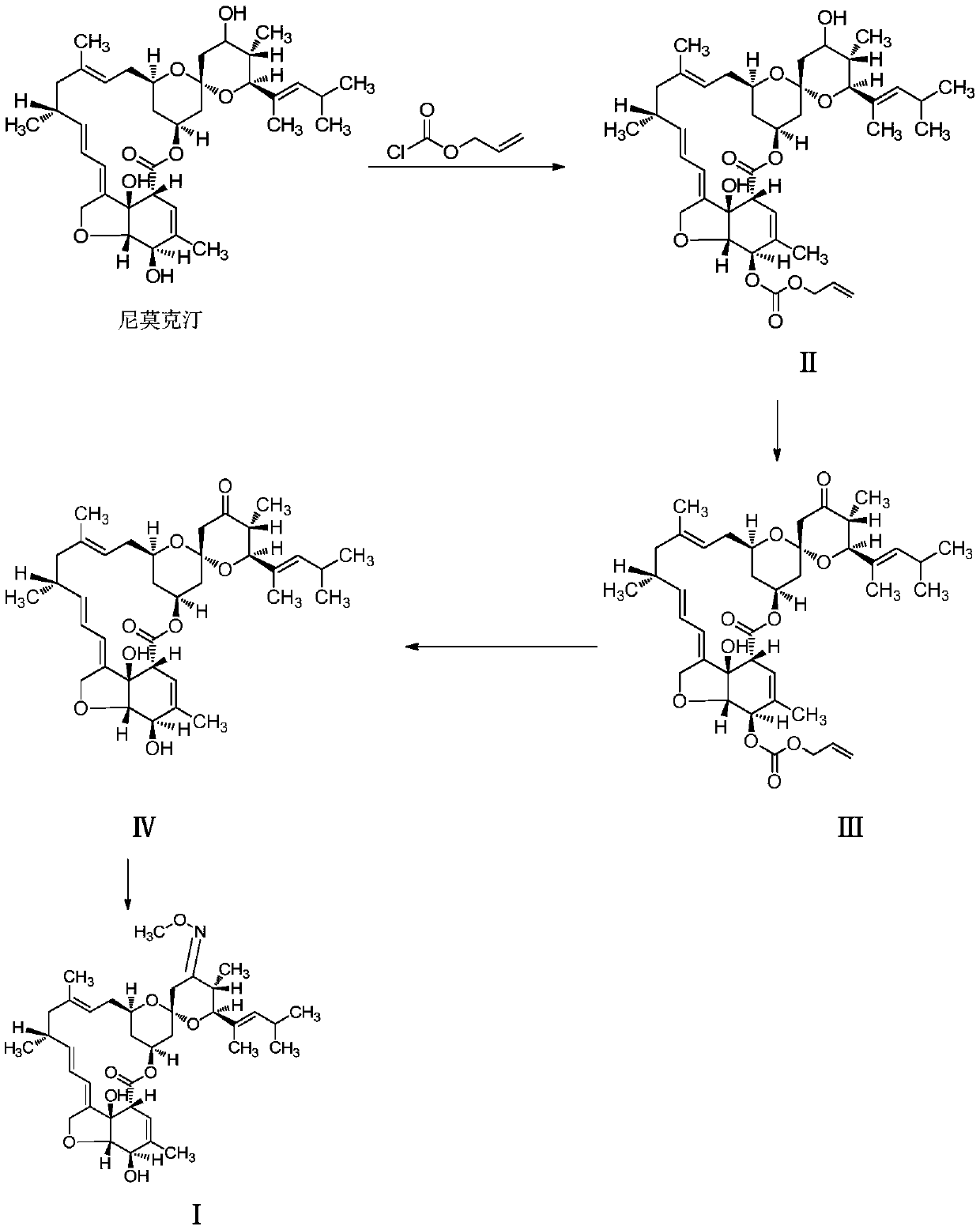
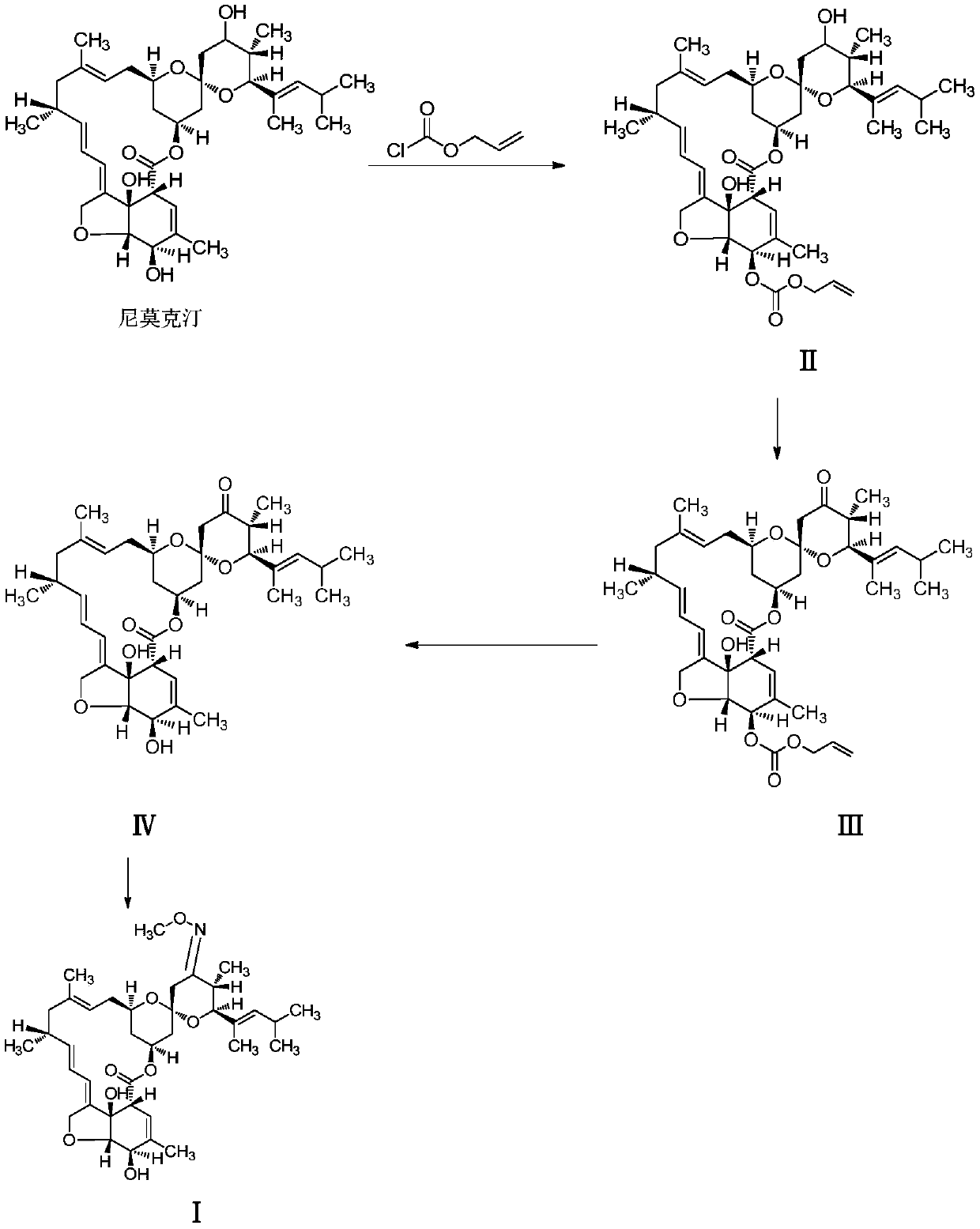
Method for preparing moxidectin
An allyloxycarbonyl and oxo technology, applied in the field of preparation of new antiparasitic drugs, can solve problems such as affecting product quality and yield, and achieve the effects of sufficient supply, mild reaction conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A. Preparation of 5-oxo(allyloxycarbonyl)nimoctin (Ⅱ)
[0048] Add nimoctine (613g, 1.0mol), imidazole (613g) and dichloromethane (3050g) into the reactor, stir well and cool to -5 to 5°C, then add allyl chloroformate dropwise under stirring (132.5g, 1.1mol) and dichloromethane (600g) mixed solution, keep the temperature of the mixture not exceeding 5°C during the dropwise addition, keep -5 to 5°C after the dropwise addition, continue to stir and react for 8 hours, and naturally rise after the reaction to room temperature, the mixture was washed with saturated brine, the organic phase was dried with anhydrous sodium sulfate after separation, the filtrate was filtered to remove the desiccant, and the filtrate was concentrated to obtain an oil, and the oil was recrystallized with methanol (2000g) to obtain 5- Oxy(allyloxycarbonyl)nemoctine (Ⅱ), 683.1g of light yellow powder, yield about 98.0%, HPLC detection is consistent with the standard comparison, content greater than...
Embodiment 2
[0056] Other steps are identical with embodiment 1, just the preparation method of the 5-oxygen (allyloxycarbonyl) nimoctine (II) of A step is as follows:
[0057] Add nimoctine (613g, 1.0mol), imidazole (367.8g) and dichloromethane (1250g) into the reactor, stir well and cool to -5 to 5°C, then add allyl chloroformate dropwise under stirring A mixed solution of ester (120.5g, 1.0mol) and dichloromethane (600g), keep the temperature of the mixture not exceeding 5°C during the dropwise addition, and keep stirring at -5 to 5°C after the dropwise addition for 4 hours. Rising to room temperature, the mixture was washed with saturated brine, the organic phase was dried with anhydrous sodium sulfate after separation, the filtrate was filtered to remove the desiccant, and the filtrate was concentrated to obtain an oily substance, which was recrystallized with methanol (1800g) to obtain 5 -Oxy(allyloxycarbonyl)nemoctine (Ⅱ), 534.6g of light yellow powder, yield about 77.0%, HPLC detec...
Embodiment 3
[0059] Other steps are identical with embodiment 1, just the preparation method of the 5-oxygen (allyloxycarbonyl) nimoctine (II) of A step is as follows:
[0060] Add nimoctine (613g, 1.0mol), imidazole (490g) and dichloromethane (2200g) into the reactor, stir well and cool to -5 to 5°C, then add allyl chloroformate dropwise under stirring (126.5g, about 1.05mol) and dichloromethane (600g) mixed solution, keep the temperature of the mixture not exceeding 5°C during the dropwise addition, keep -5 to 5°C after the dropwise addition and continue to stir the reaction for 6 hours. Rising to room temperature, the mixture was washed with saturated brine, the organic phase was dried with anhydrous sodium sulfate after separation, the filtrate was filtered to remove the desiccant, and the filtrate was concentrated to obtain an oil, and the oil was recrystallized with methanol (2500g) to obtain 5 -Oxy(allyloxycarbonyl)nemoctine (II), 608.5g of light yellow powder, yield about 87.3%, HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com