Long-acting brexpiprazole in-situ phase change gel injection and preparation method thereof
A gel injection, epipiprazole technology, applied in the field of pharmaceutical preparations, can solve the problems of limited release time, poor stability, poor injectability, etc., and achieves low equipment requirements, excellent fluidity and injectability, and operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of long-acting injectable ebiprazole phase-change gel based on soybean lecithin
[0042] Prescription screening: with MCT (medium chain triglyceride), GDO (glyceryl dioleate), Span-80, ethyl oleate, PVP (polyvinylpyrrolidone), propylene glycol, Tween-80 as additives, Using ethanol, aqueous ethanol and NMP (N-methyl-2-pyrrolidone) as solvents, the formulation of phase change gel based on soybean lecithin (model: S100) was screened. The screening prescription is shown in Table 1 below, and the unit of each component is "g".
[0043]
[0044] Preparation method: Weigh the ebiprazole raw material medicine of the prescription amount in Table 1, add solvents such as NMP and ethanol of various concentrations corresponding to the prescription amount, and dissolve the ebiprazole; then, add S100 soybean lecithin to the obtained solution in sequence And viscosity-increasing additives such as MCT, GDO, ethyl oleate, Pansi 80, PVP, propylene glycol and T...
Embodiment 2
[0049] Example 2 : Ebiprazole phase change gel injection based on egg yolk lecithin
[0050] Prescription screening: with MCT (medium chain triglyceride), GDO (glyceryl dioleate), Span-80, ethyl oleate, PVP (polyvinylpyrrolidone), propylene glycol, Tween-80 as additives, Ethanol, aqueous ethanol and NMP (N-methyl-2-pyrrolidone) were used as solvents to screen the formulation of phase change gel based on egg yolk lecithin (model: PL-100M). The screening prescription is shown in Table 4, and the unit of each component is "g".
[0051]
[0052] Preparation process: according to the preparation method of Example 1, first weigh the ebiprazole bulk drug in the prescription amount in Table 4, add the solvent NMP corresponding to the prescription amount or ethanol of different concentrations to dissolve it, and obtain the ebiprazole solution. Subsequently, add PL-100M egg yolk lecithin and viscosity-increasing additives such as MCT, GDO, soybean oil, Pansi 80, PVP, propylene gly...
Embodiment 3
[0056] Example 3 : Solubility of Ebiprazole in Phospholipid Gels
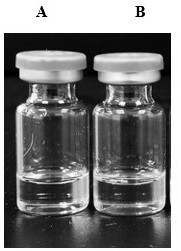
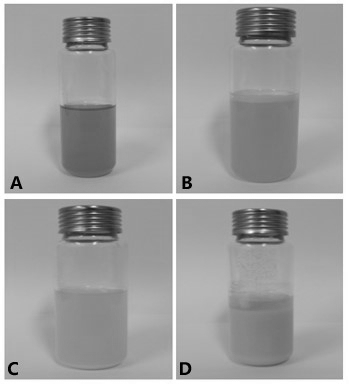
[0057] The appearance photos of two kinds of ebiprazole in-situ phase-change gel injections A and B prepared according to Example 1 and Example 2 are as follows: figure 1 shown. By comparison, it can be found that the appearance of the ebiprazole phase-change gel long-acting injection of the present invention and the gel injection disclosed in patents CN102526753B, CN107049932A, CN102008728B and CN104661648B (see figure 2 ) has a significant difference, which is mainly manifested in the different solubility of insoluble active ingredients, see Table 7.
[0058]
[0059] The results in Table 7 show that the phase-change gel injections of the present invention are all clear and transparent solutions, and the insoluble active ingredient ebiprazole can be completely dissolved, and there is no precipitation or crystal precipitation after standing, even after centrifugation at 4000rpm for 5min. No delaminatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity value | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com