Composition capable of reducing uric acid and resisting gout, and preparation method and application of composition
An anti-gout and uric acid-lowering technology, applied to a composition with anti-gout function and preparation thereof, has the field of uric acid lowering, and can solve the problems of inability to enhance the inhibition of uric acid generation, limited inhibition of uric acid generation, and lowering of blood uric acid levels, and the like. The effect of inhibiting uric acid production, increasing uric acid excretion, and reducing blood uric acid levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
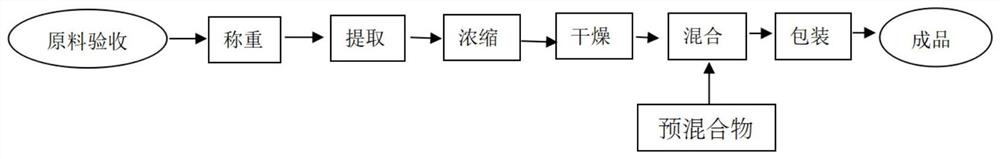
Image
Examples
Embodiment 1
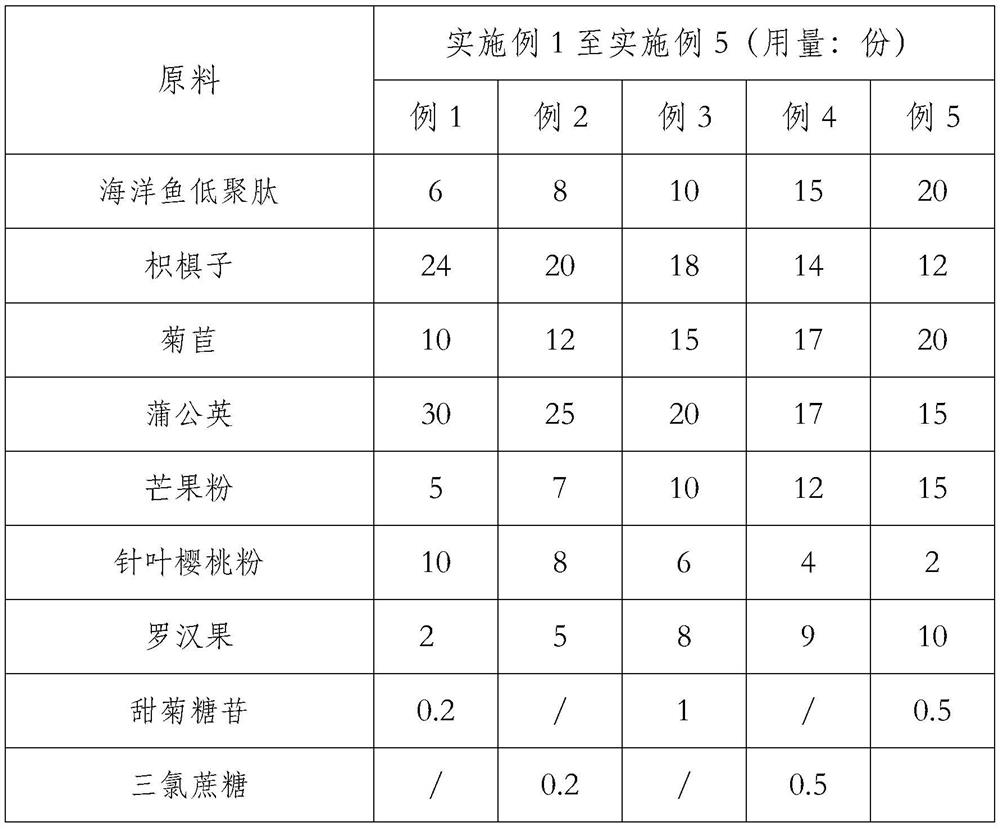
[0040] 100 parts of the uric acid-lowering and anti-gout composition described in this example are made of the following raw materials in parts by weight: 6 parts of marine fish oligopeptides, 24 parts of Hovenia dulcis, 10 parts of chicory, 30 parts of dandelion, and 5 parts of mango powder , 10 parts of acerola cherry powder, 2 parts of monk fruit, 0.2 part of steviol glycoside, and the rest is maltodextrin. [Here, the maltodextrin is represented by the remaining amount of parts. In the middle of the claim, it is 5%-10% maltodextrin in the concentrated solution, so it is easy to cause doubts: 5%-10% maltodextrin in the concentrated solution Is it necessarily corresponding to the remaining amount? It is suggested that the two should be unified. For example, in claim 1, the number of parts of maltodextrin is limited, and it will not be expressed according to the volume ratio in the subsequent concentrated solution]
[0041] The preparation method of this embodiment is as follo...
Embodiment 2
[0048] Embodiment 2: 100 parts of the uric acid-lowering and anti-gout composition described in this embodiment are made of the following raw materials in parts by weight: 8 parts of marine fish oligopeptides, 20 parts of Hovenia dulcis, 12 parts of chicory, 25 parts of dandelion, 7 parts of mango powder, 8 parts of acerola powder, 5 parts of monk fruit, 0.2 part of sucralose, and the rest is maltodextrin.
[0049] The preparation method of this embodiment is as follows:
[0050] Step (1), the raw material acceptance of the composition of the present invention, the marine fish oligopeptide used is checked and accepted according to the national standard GBT22729-2008; the content of mangiferin in mango powder ≥ 100mg / 100g, and the content of VC in acerola cherry ≥ 60%; according to "China The Monk Fruit standard in Pharmacopoeia is accepted; steviol glycoside is accepted according to GB 8270 national food safety standard; sucralose is accepted according to GB 25531 national foo...
Embodiment 3
[0056] Embodiment 3: 100 parts of the uric acid-lowering and anti-gout composition described in this embodiment are made of the following raw materials in parts by weight: 10 parts of marine fish oligopeptides, 18 parts of Hovenia dulcis, 15 parts of chicory, 20 parts of dandelion, 10 parts of mango powder, 6 parts of acerola cherry powder, 8 parts of Luo Han Guo, 1 part of steviol glycoside, and the rest is maltodextrin.
[0057] The preparation method of this embodiment is as follows:
[0058] Step (1), the raw material acceptance of the composition of the present invention, the marine fish oligopeptide used is checked and accepted according to the national standard GBT22729-2008; the content of mangiferin in mango powder ≥ 100mg / 100g, and the content of VC in acerola cherry ≥ 60%; according to "China The Monk Fruit standard in Pharmacopoeia is accepted; steviol glycoside is accepted according to GB 8270 national food safety standard; sucralose is accepted according to GB 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com