Sucrose phosphorylase mutant and application thereof
A phosphorylase mutant, sucrose phosphorylase technology, applied in the field of enzyme engineering and microbial engineering, can solve problems such as poor thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Expression of sucrose phosphorylase wild enzyme
[0044](1) Obtain the gene sequence of the SPase gene (GenBank accession number: D90314) of Leuconostoc enteritidis ATCC 12291 from NCBI, optimize it according to the codon preference of E. NcoI and XhoI were added to both ends of the optimized SPase gene, and sent to Jinweizhi Biotechnology Co., Ltd. to synthesize the sucrose phosphorylase whose coding nucleotide sequence is shown in SEQ ID NO.1.
[0045] (2) Construction and transformation of gene expression vector
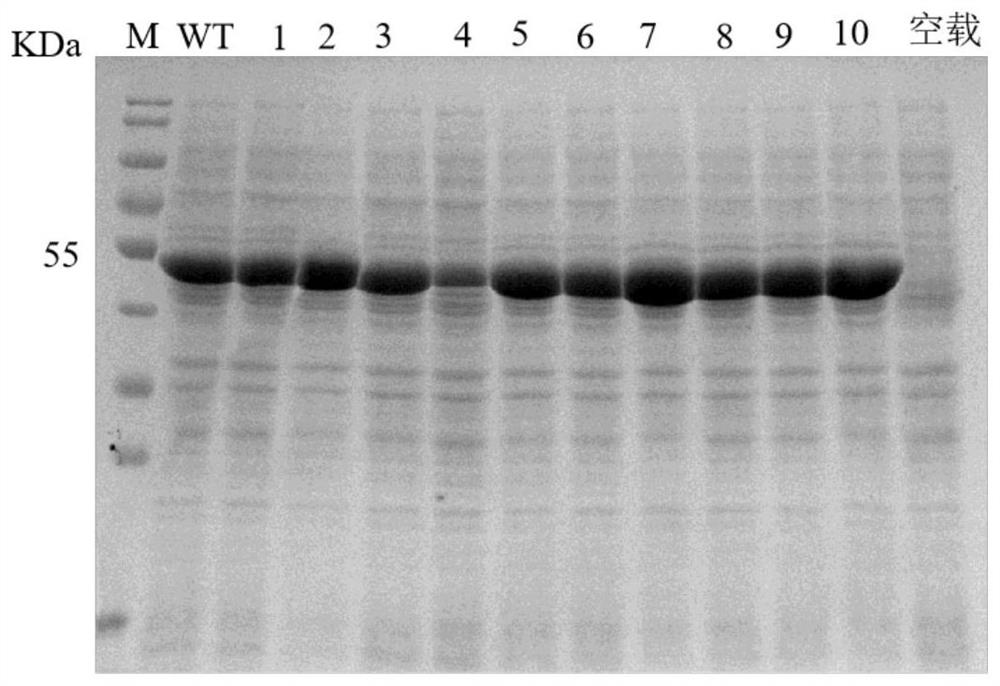
[0046] The sucrose phosphorylase SPase and the pET-28a vector whose coding nucleotide sequence is shown in SEQ ID NO.1 are double-digested with restriction endonucleases NcoI and XhoI, and the digested product is connected with Solution I to obtain a recombinant vector pET-28a-SPase, and then transfer the recombinant vector pET-28a-SPase into Escherichia coli BL21 (DE3) for expression, pick 4 transformants to extract the plasmid and verify it wi...
Embodiment 2
[0047] Example 2: Preparation and expression of sucrose phosphorylase mutants
[0048] Specific steps are as follows:
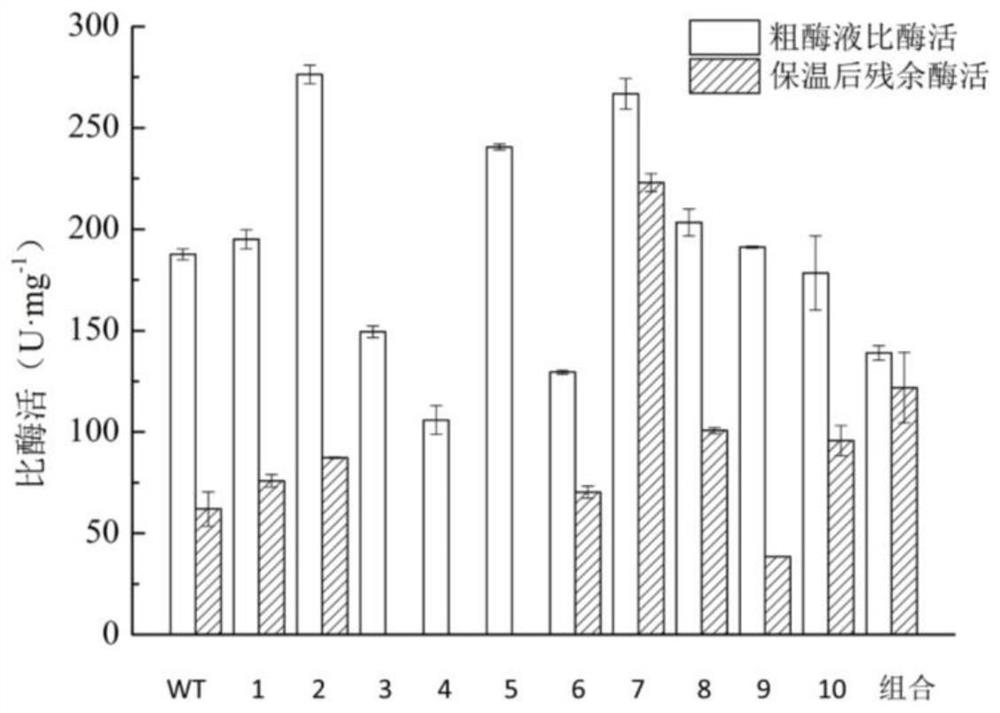
[0049] Utilizing the whole plasmid PCR technology, the recombinant plasmid pET-28a-spase obtained in Example 1 was used as a template for site-directed mutation, and mutants I31F, Q453G, G252L, A232M, T152G, N158C, T219L, S360A, N249A, T263L and T31F\T219L\S360A\T263L gene recombinant plasmids pET-28a-SPase1~pET-28a-SPase11;
[0050] Wherein, the mutation I31F is obtained by mutating the 31st amino acid of the sucrose phosphorylase shown in SEQ ID NO.22 from isoleucine to phenylalanine, and the primers used are as follows:
[0051] I31F-1: 5'-GTTCTGAAAGAAGAC TTC GGTGACGCTA-3' (SEQ ID NO. 2);
[0052] I31F-2: 5'-ACCGATAGCG TCACC GAA GTCTTCTTTC-3' (SEQ ID NO. 3);
[0053] The mutation Q453G is obtained by mutating the 453rd amino acid of sucrose phosphorylase with the amino acid sequence shown in SEQ ID NO.22 from glutamine to glycine, and the primers us...
Embodiment 3
[0093] Embodiment 3: Separation and purification of different sucrose phosphorylase mutants
[0094] Specific steps are as follows:
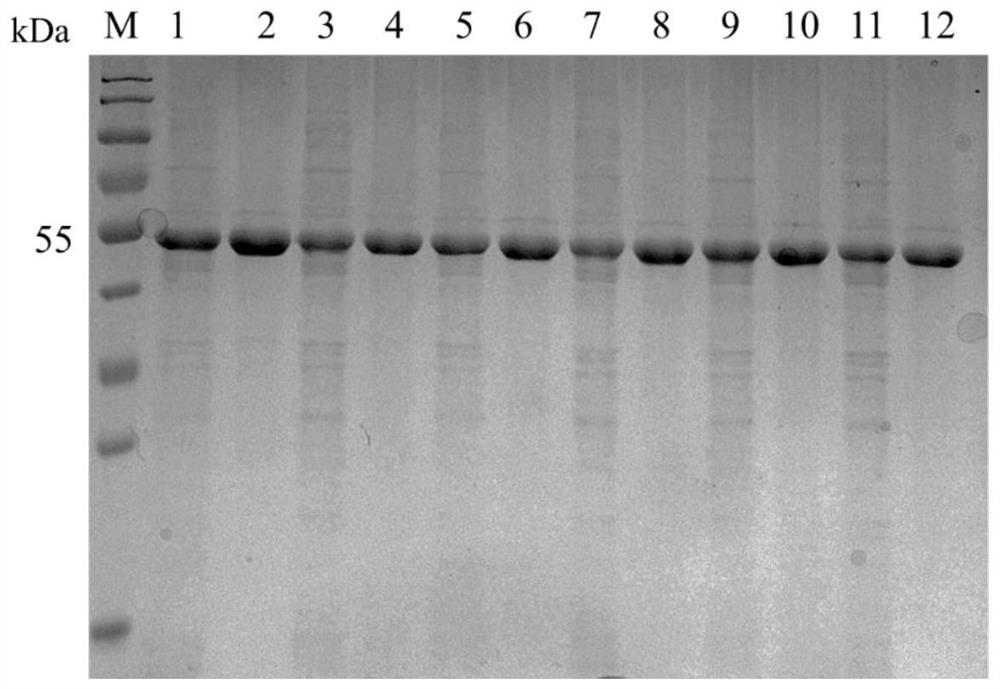
[0095] The crude enzyme solutions containing sucrose phosphorylase mutants I31F, T219L, S360A, T263L and T31F\T219L\S360A\T263L obtained in Example 2 were purified respectively, and a His tag was attached to the SPase C-terminus, and the nickel column affinity Proteins were purified by chromatography.
[0096] (1) Prepare fresh crude enzyme solutions containing sucrose phosphorylase mutants I31F, T219L, S360A, T263L and T31F\T219L\S360A\T263L in advance and place them on ice (the purification process should be carried out at low temperature as much as possible);
[0097] (2) Use binding buffer at 1 mL·min -1 The column was equilibrated at a flow rate, and then the crude enzyme solution containing sucrose phosphorylase mutants I31F, T219L, S360A, T263L and T31F\T219L\S360A\T263L was mixed at 0.5mL·min -1 Load the sample at a flow rate, and was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com