A kind of synthetic method of ibrutinib
A synthetic method, the technology of ibrutinib, applied in the field of drug synthesis, can solve the problems of waste of raw materials, industrial production costs, low purity affecting product quality, and insufficient purity and yield of ibrutinib, so as to reduce waste of raw materials and industrial production. Production cost, high purity and yield, and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Intermediate M3 was prepared according to the following steps:
[0030] (1) Mix and stir 25g 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidine, 250mLTHF and 43.2g triphenylphosphine to dissolve, add 35g (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, stirred for 10min;
[0031] (2) Protect from light, control the temperature at 10°C, start adding diisopropyl azodicarboxylate dropwise, and stir for 2 hours; cool the reaction down to 0°C, add concentrated hydrochloric acid, adjust the pH of the reaction system to 1, and then raise the temperature to 40°C Reaction 3h;
[0032] (3) Add 500ml of purified water, wash with 500ml of ethyl acetate for 5 times to remove impurities;
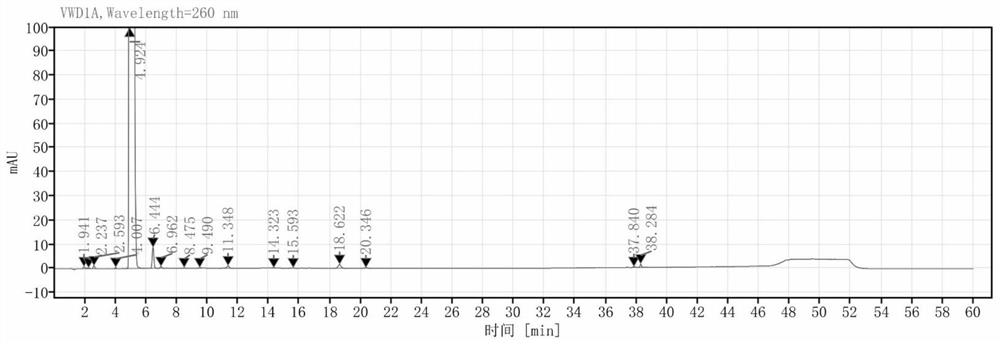
[0033] (4) Add NaOH aqueous solution with a mass fraction of 25% dropwise in the impurity-removed system, adjust the pH=9-10, and obtain the light yellow powder intermediate M3 after desalting and crystallizing for 2 hours. figure 1 It is the HPLC spectrogram of the intermediate M3 prepared ...
Embodiment 2
[0039] Intermediate M3 was prepared according to the following steps:
[0040] (1) Mix and stir 25g 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidine, 250mLTHF and 43.2g triphenylphosphine to dissolve, add 35g (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, stirred for 30min;
[0041] (2) Protect from light, control the temperature at 15°C, start adding diisopropyl azodicarboxylate dropwise, and stir for 4 hours; cool the reaction down to 0°C, add concentrated hydrochloric acid, adjust the pH of the reaction system to 1.5, and then raise the temperature to 45°C Reaction 5h,
[0042] (3) Add 750mL of purified water, wash 5 times with 600ml of ethyl acetate to remove impurities,
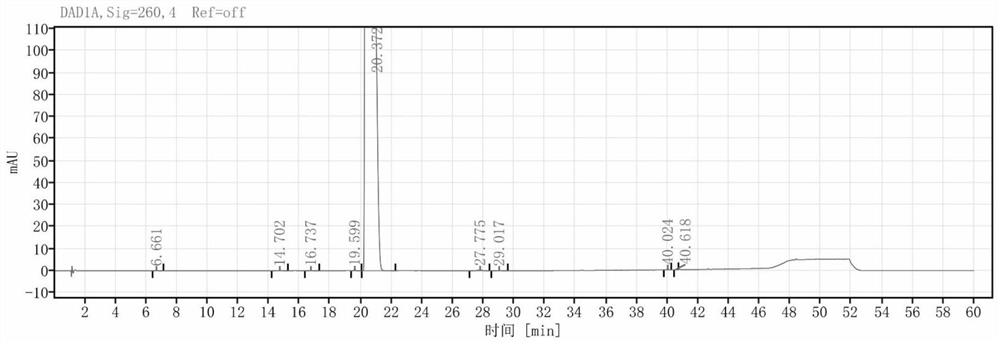
[0043] (4) Add NaOH aqueous solution with a mass fraction of 25% dropwise to the impurity-removed system, adjust the pH=10, desalt and crystallize for 2 hours to obtain a light yellow powder intermediate M3 with a purity of 99.011%
[0044] Ibrutinib is prepared according to the following step...
Embodiment 3
[0049] Intermediate M3 was prepared according to the following steps:
[0050] (1) Mix and stir 25g 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidine, 250mLTHF and 43.2g triphenylphosphine to dissolve, add 35g (S)-tert-butyloxycarbonyl-3-hydroxypiperidine, stirred for 20min;
[0051] (2) Protect from light, control the temperature at 10-15°C, start adding diisopropyl azodicarboxylate dropwise, and stir for 2 hours; cool the reaction down to 0°C, add concentrated hydrochloric acid, adjust the pH of the reaction system to 1, and then raise the temperature to React at 42°C for 4h,
[0052] (3) Add 600mL of purified water, wash 5 times with 550ml of ethyl acetate to remove impurities,
[0053] (4) Add NaOH aqueous solution with a mass fraction of 25% dropwise to the impurity-removed system, adjust the pH=10, desalt and crystallize for 2 hours to obtain a light yellow powder intermediate M3 with a purity of 99.101%
[0054] Ibrutinib is prepared according to the following...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com