Preparation method of levonorgestrel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
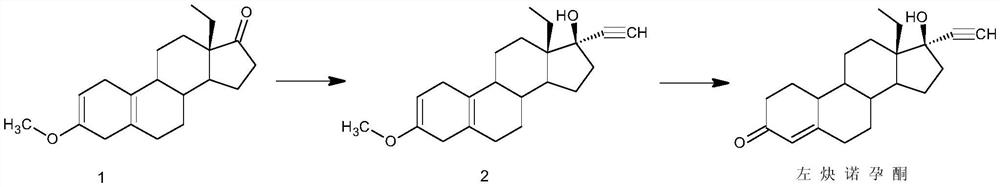
[0025] Embodiment 1 A kind of preparation method of levonorgestrel comprises the steps:
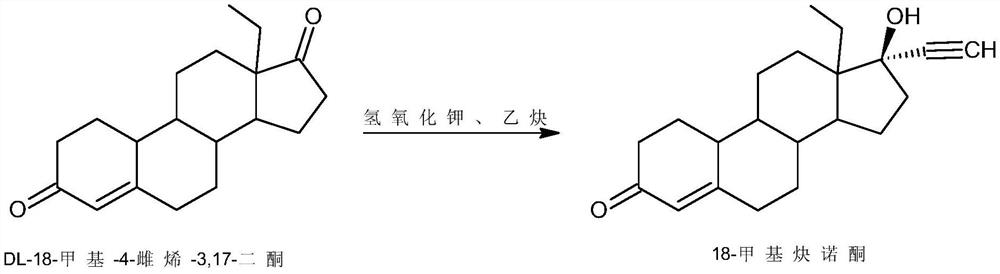
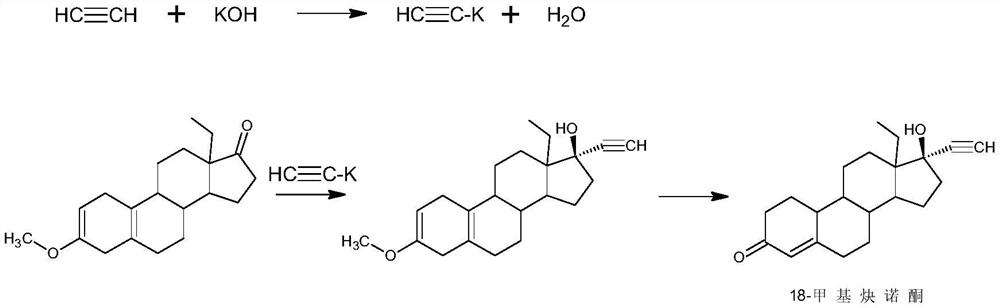
[0026] 1) Alkynylation: Dissolve 50g of 18-methylestro-2,5(10)-diene-3-methoxy-17-one (1) in 750ml of THF, add 50g of potassium tert-butoxide, and control the temperature Pass through acetylene at 5°C, stir the reaction, after the reaction, add 5% hydrochloric acid aqueous solution to neutralize to neutral, concentrate, add water for water analysis, filter to obtain 52g of intermediate 2;
[0027] 2) Hydrolysis reaction: 52g of intermediate (2) obtained in step 1) was dissolved in 1500ml of acetone, 250ml of 5% hydrochloric acid aqueous solution was added, and reacted at 0°C. After the reaction, 10% sodium carbonate aqueous solution was added to neutralize to neutrality, Concentrate, add water for water analysis, and filter to obtain a crude product. The crude product is refined with methanol and dried to obtain 43 g of levonorgestrel. The total mass yield of the product is 86%, and the H...
Embodiment 2
[0028] Embodiment 2 A kind of preparation method of levonorgestrel, comprises the steps:
[0029] 1) Alkynylation reaction: 50g of 18-methylestro-2,5(10)-diene-3-methoxy-17-one (1) was dissolved in 250ml of dimethyl sulfoxide, and 100g of isopropanol was added Potassium, control the temperature at 30°C and pass through acetylene, stir the reaction, after the reaction, add 10% sulfuric acid aqueous solution A to neutralize to neutral, concentrate, add water for water analysis, filter to obtain 53g of intermediate 2;
[0030] 2) Hydrolysis reaction: Dissolve 53g of intermediate (2) obtained in step 1) in 250ml of tetrahydrofuran, add 27ml of 30% aqueous hydrochloric acid, and react at -10°C. After the reaction, add 5% aqueous sodium hydroxide to neutralize Neutral, concentrated, added water for water analysis, filtered to obtain a crude product, which was refined with ethanol and dried to obtain 43.5 g of levonorgestrel with a total mass yield of 87% and an HPLC content of 99.2%...
Embodiment 3
[0031] Embodiment 3 A kind of preparation method of levonorgestrel, comprises the steps:
[0032] 1) Alkynylation reaction: 50g of 18-methylestr-2,5(10)-diene-3-methoxy-17-one (1) was dissolved in 1500ml of toluene, 250g of sodium ethylate was added, and the temperature was controlled at -20 Pass through acetylene at ℃, stir the reaction, after the reaction, add 30% acetic acid aqueous solution to neutralize to neutral, concentrate, add water for water analysis, filter to obtain 51g of intermediate 2;
[0033] 2) Hydrolysis reaction: Dissolve 51 g of intermediate (2) obtained in step 1) in 750 ml of butanone, add 70 ml of 10% sulfuric acid aqueous solution, and react at 50 ° C. After the reaction, add 30% sodium sulfite aqueous solution to neutralize , concentrated, added water for water analysis, filtered to obtain a crude product, which was refined with acetone and dried to obtain 43g of levonorgestrel, with a total mass yield of 86% and an HPLC content of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com