Dehydroabietic acid-based 2,4-diarylbenzimidazole fluorescent probe for ferric ions and mercury ions, and preparation method and application thereof
A technology of benzimidazole and fluorescent probes, applied in fluorescence/phosphorescence, organic chemical methods, chemical instruments and methods, etc., can solve the problems of high professionalism in operation and strict detection environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthetic method of dehydroabietic acid base 2,4-diarylbenzimidazole compound, the synthetic process is:
[0032]
[0033] Specific steps are as follows:
[0034] 1) Preparation of 12-bromo-13,14-dinitrodehydroabietic acid methyl ester: dehydroabietic acid is prepared through methyl esterification, bromination and double nitration reactions. The specific process is as follows:
[0035] Weigh 30g of dehydroabietic acid and dissolve it in 60mL of toluene, add 11mL of thionyl chloride and react at 78-80°C for 3h, spin dry under vacuum, add 60mL of methanol, react at 78-80°C for 3h, spin dry and add 30mL of ethanol was dissolved to obtain methyl dehydroabietate;
[0036] Accurately weigh 5g of methyl dehydroabietate, dissolve it in 30mL of acetonitrile, add 4g of N-bromosuccinimide, and react at room temperature in the dark for 24 hours, rotary steam, wash twice with dichloromethane, dissolve crystals in 100mL of methanol, Obtain 12-bromodehydroabietic acid methyl ...
Embodiment 2
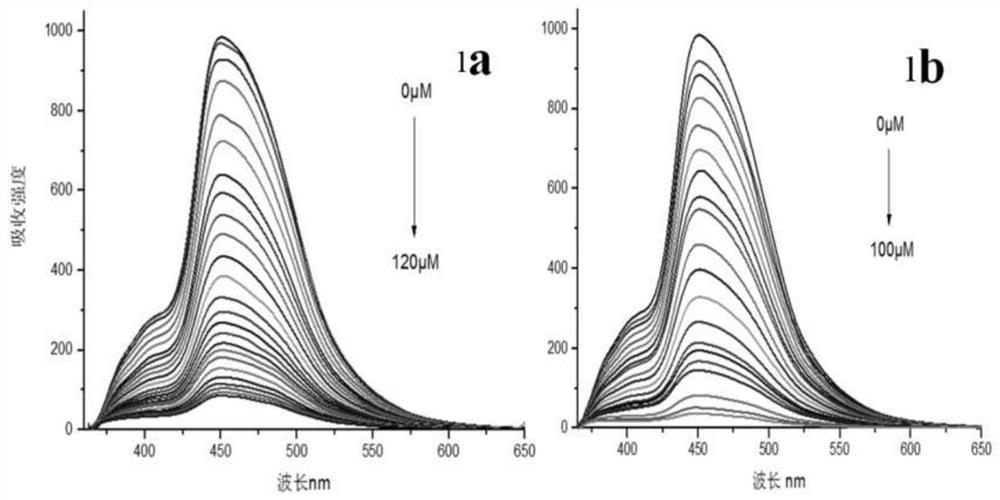
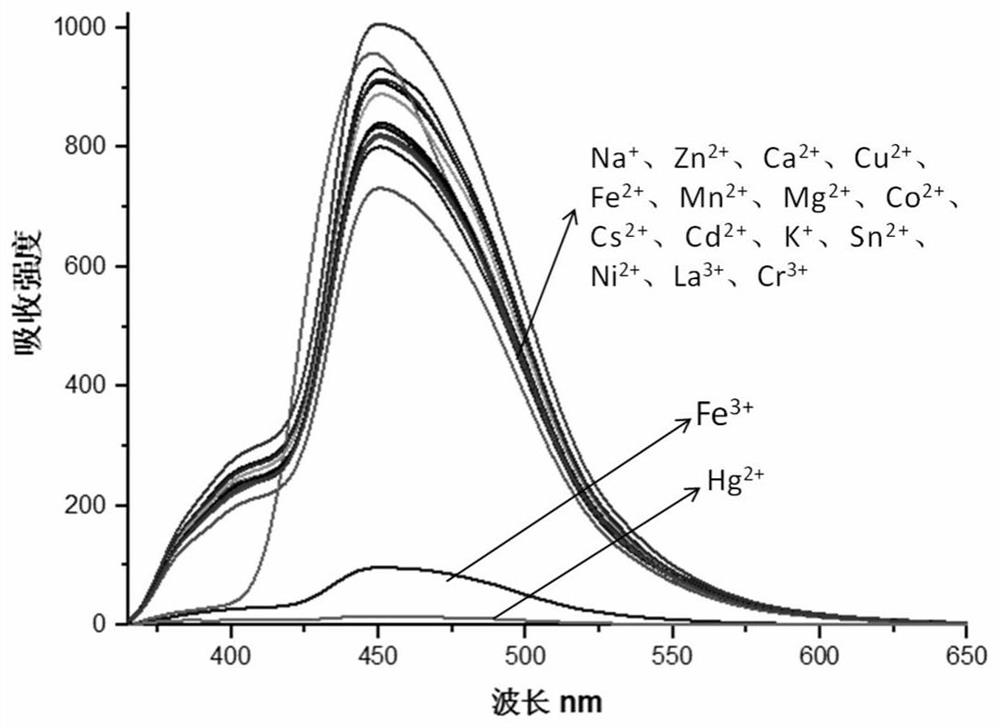
[0046] 2-(2-Hydroxynaphthalen-1-yl)-6,9a-dimethyl-11-(3,4,5-methoxyphenyl-4,5,5a,6,7,8,9 ,9a-octahydro-3H-phenanthro[1,2-d]imidazole-6-methyl ester was dissolved in aqueous ethanol (ethanol:water=1:1) (1×10 -5 M), add (0-1)×10 -4 Fe of M 3+ , Hg 2+ Different concentrations of Fe were measured 3+ , Hg 2+ p-2-(2-Hydroxynaphthalen-1-yl)-6,9a-dimethyl-11-(3,4,5-methoxyphenyl-4,5,5a,6,7,8,9 , the fluorescence absorption spectrum of 9a-octahydro-3H-phenanthro[1,2-d]imidazole-6-methyl ester, such as figure 11a: the fluorescence absorption spectrum of the addition of iron ions; 1b: the fluorescence absorption spectrum of the addition of mercury ions, the fluorescence absorption of the compound is obviously weakened until it is quenched, indicating that the compound can interact with Fe 3+ , Hg 2+ Complexation.
Embodiment 3
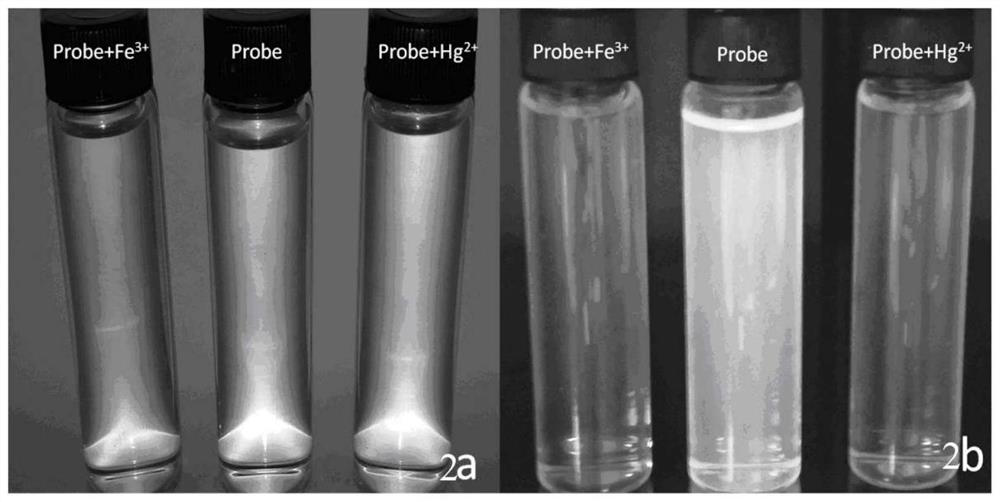
[0048] Accurately weigh 62mg of 2-(2-hydroxynaphthalen-1-yl)-6,9a-dimethyl-11-(3,4,5-methoxyphenyl-4,5,5a,6,7, 8,9,9a-Octahydro-3H-phenanthro[1,2-d]imidazole-6-methyl ester was dissolved in 10mL of absolute ethanol to prepare 1×10 -3 M solution, take 100 μM stock solution and dilute it with ethanol aqueous solution (ethanol:water=1:1) to 10mL with a concentration of 1×10 -5 M solution, 5 μL of Fe was added each time 3+ (1×10 -2 M), Hg 2+ (1×10 -2 M), with Fe 3+ The added solution turns pale yellow, with the increase of Hg 2+ The color of the added solution did not change. Observed under 365nm ultraviolet light, such as figure 2 As shown, 2a: the compound added Fe under sunlight 3+ , Hg 2+ Photos before and after ions; 2b: Fe added to the compound under 365nm UV light 3+ , Hg 2+ Ion before and after photos with Hg added 2+ , Fe 3+ The blue fluorescence of the solution of ions is quenched, indicating that the compound is compatible with Hg 2+ , Fe 3+ Complexation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com