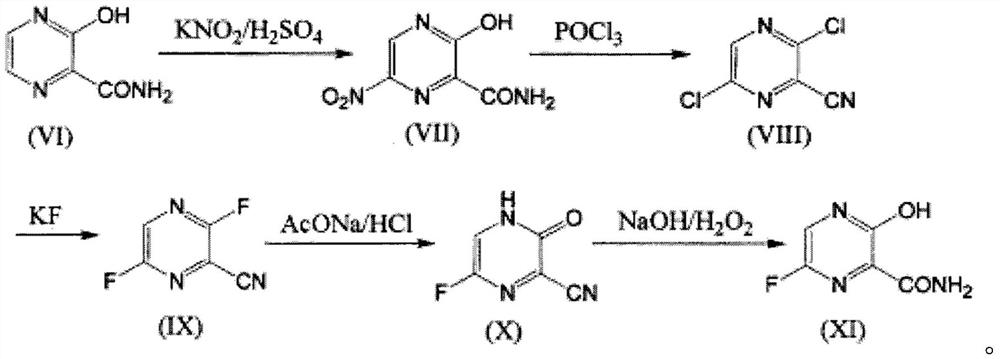
Preparation method of favipiravir
A technology of favipiravir and hydroxyl, applied in the new preparation field of favipiravir, can solve the problems of unsafe operation, unfriendly environment, large amount of waste water, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
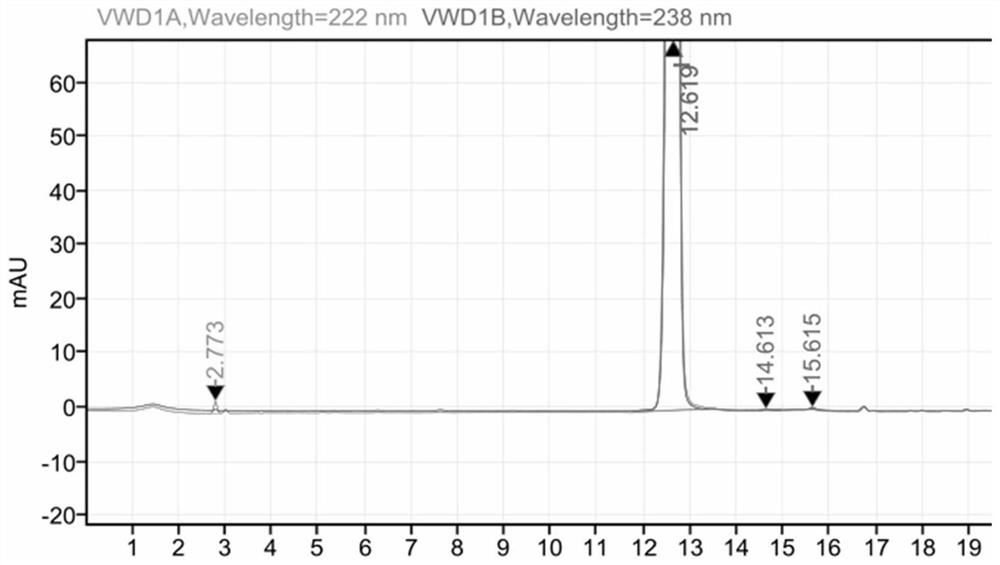
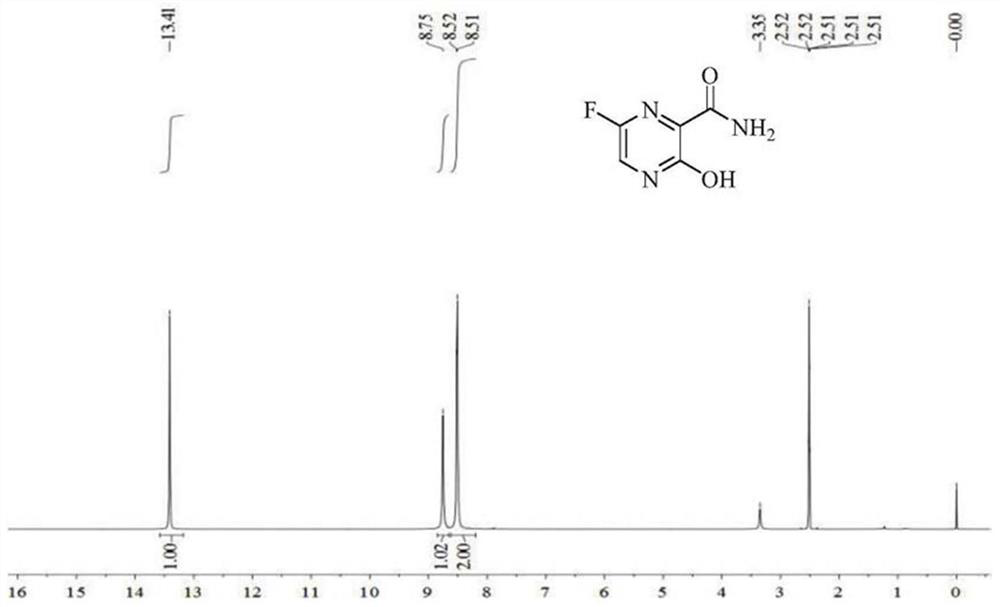
[0022] Add 30 grams of 6-fluoro-3-hydroxy-2-cyanopyrazine dicyclohexylamine salt to 100 grams of anhydrous tert-butanol, and then add 22.4 grams of potassium hydroxide. The temperature was raised to 60° C., and the reaction was carried out for 8 hours. TLC detected that the reaction of the raw materials was complete. Evaporate tert-butanol, add 150 ml of water, 100 ml of ethyl acetate, adjust the pH of the water phase to 5-6 with hydrochloric acid, stir, let stand to separate layers, separate the ethyl acetate layer, wash once with 100 ml of saturated saline, and wash with Dry over anhydrous magnesium sulfate, concentrate under reduced pressure to obtain 14.13 grams of product, nuclear magnetic pattern is as follows figure 2 , it can be determined that it is Favipiravir. The molar yield is 96%, and the purity is 99.9%.
Embodiment 2
[0024] Add 27.8 grams of 6-fluoro-3-hydroxy-2-cyanopyrazine to 150 grams of anhydrous isopropanol, and then add 32 grams of sodium hydroxide. The temperature was raised to 60° C., and the reaction was carried out for 8 hours. TLC detected that the reaction of the raw materials was complete. Evaporate tert-butanol, add 150 ml of water, 150 ml of ethyl acetate, adjust the pH of the water phase to 5-6 with hydrochloric acid, stir, let stand to separate layers, separate the ethyl acetate layer, wash once with 150 ml of saturated saline, and wash with Dried over anhydrous magnesium sulfate, concentrated under reduced pressure to obtain 29.85 g of the product, the NMR results were the same as in Example 1, and it could be determined that it was favipiravir; the molar yield was 95%, and the purity was 99.9%.
Embodiment 3
[0026] Add 30 grams of 6-fluoro-3-hydroxy-2-cyanopyrazine dicyclohexylamine salt to 360 grams of anhydrous tert-butanol, and then add 180 grams of potassium hydroxide. The temperature was raised to 30° C., and the reaction was carried out for 8 hours. TLC detected that the reaction of the raw materials was complete. Evaporate tert-butanol, add 150 ml of water, 100 ml of ethyl acetate, adjust the pH of the water phase to 5-6 with hydrochloric acid, stir, let stand to separate layers, separate the ethyl acetate layer, wash once with 100 ml of saturated saline, and wash with Drying over anhydrous magnesium sulfate, concentrating under reduced pressure, the NMR results of the product obtained are the same as in Example 1, and it can be determined that it is favipiravir; the molar yield is 98%, and the purity is 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com