Benzothiophene compound as well as preparation method and application method thereof
A technology of benzothiophene and an application method, which is applied in the field of heterocyclic compound synthesis, can solve the problems of high environmental hazard of halogen-containing waste liquid, does not conform to green chemistry, harsh reaction conditions, etc., achieves good scalability, reduces synthesis cost, and reacts The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Preparation of benzothiophene compound 1
[0048] 2-Phenylethynylanisole sulfide (44.8mg, 0.2mmol), DTPB (117.0mg, 0.8mmol), disodium hydrogen phosphate (283.9mg, 0.2mmol), acetone (2mL), Under the protection of nitrogen, raise the temperature to 130°C and stir the reaction for 24 hours, extract with 2x8mL dichloromethane, combine the organic phases, dry overnight with anhydrous sodium sulfate or anhydrous magnesium sulfate, spin dry, and pass through the column with petroleum ether: ethyl acetate = 2:1 , to obtain 36.2 mg of the target compound as a white solid, with a yield of 68%.
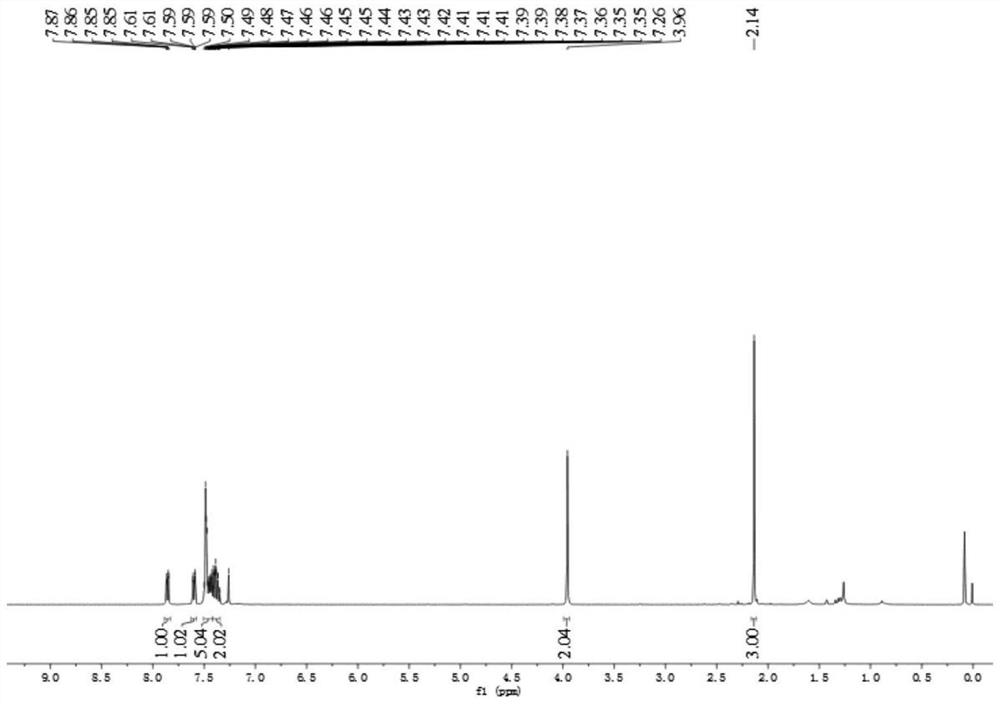
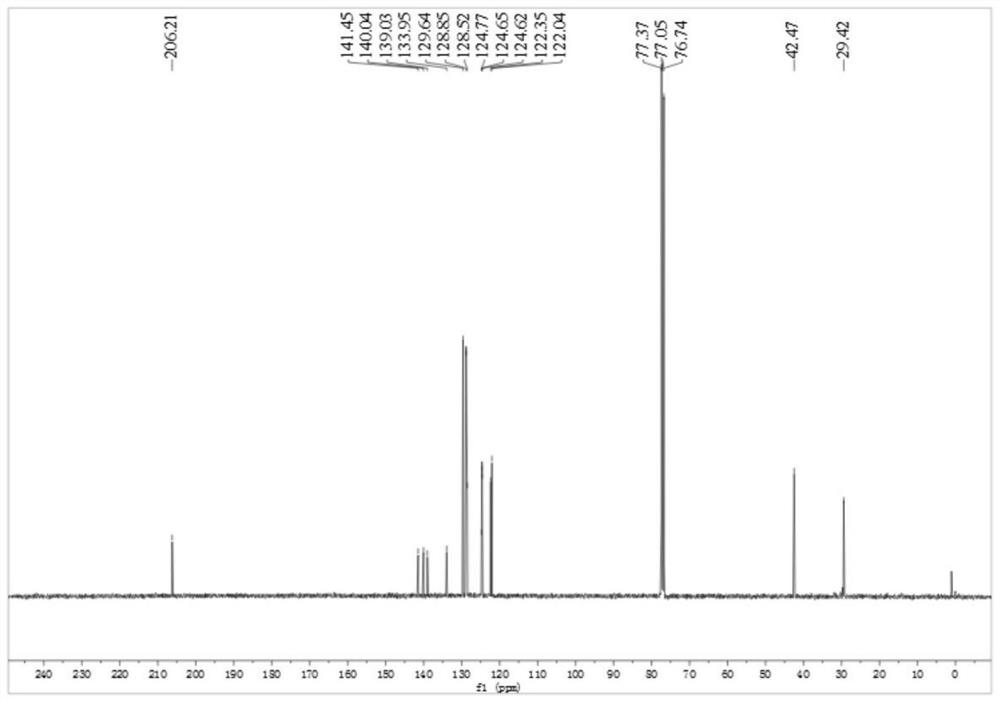
[0049] Product structure verification (see attached Figures 1A-1B ):
[0050] 1 H NMR (δ, ppm, 400MHz, CDCl 3 ):δ7.86(dd,J=7.4,1.6Hz,1H),7.60(dd,J=7.4,1.6Hz,1H),7.52–7.42(m,5H),7.42–7.34(m,2H), 3.96(s,2H),2.14(s,3H);
[0051] 13 C NMR (δ, ppm, 100MHz, CDCl 3 ): δ206.2, 141.5, 140.0, 139.0, 133.9, 129.6, 128.8, 128.5, 124.8, 124.65, 124.62, 122.3, 122.0, 42.5, 29.4. ...
Embodiment 2
[0052] Embodiment 2: the selection of free radical initiator consumption
[0053] The experimental conditions and feeding amount of this embodiment are the same as those in Example 1, and different amounts of free radical initiators are selected for experimentation, as shown in Table 1.
[0054] The reaction result of the free radical initiator of different consumptions of table 1
[0055]
[0056]
[0057] It can be seen from Table 1 that the reaction effect and yield of DTPB in an amount of 4-6 equiv are better, and the amount of DTPB is preferably 4 equiv in comprehensive consideration.
Embodiment 3
[0058] Embodiment 3: the selection of disodium hydrogen phosphate consumption
[0059] The experimental conditions and the feeding amount of this embodiment are the same as those in Example 1, and different amounts of disodium hydrogen phosphate are selected for the experiment, as shown in Table 2.
[0060] Table 2 Na in different amounts 2 HPO 4 The result of the reaction
[0061] serial number Na 2 HPO 4 (x equiv)
[0062] It can be seen from Table 2 that the reaction effect and yield of disodium hydrogen phosphate in an amount of 1 to 4 equiv are better, and 1 equiv is preferred in comprehensive consideration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com