Honokiol-chlorambucil co-prodrug with lymphocytic leukemia resisting effect, preparation method and application thereof
A technology of chlorambucil and prodrugs, which is applied in the preparation of organic compounds, medical preparations of non-active ingredients, and preparation of cyanide reactions. It can solve the problems of rare clinical research and achieve enhanced mitochondrial activity. , the effect of delaying the growth in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] About 180 mg of CBL (0.59 mmol) dissolved in 5 ml of DMF was added to EDCI (~135 mg, 0.70 mmol), then the solution was stirred at room temperature for 10 min; then HN (75 mg, 0.28 mmol) was added, and the reaction mixture was stirred at room temperature Stir overnight; add 50ml of ethyl acetate to the reaction solution, wash once with 50ml of water, dry with sodium sulfate, filter and concentrate; purify the yellow solid by chromatography, which is the co-precursor of honokiol-chlorambucil Drug (HN-CBL), yield 10%. 1H-NMR (400MHz, CDL3): δ1.85-1.86(m, 1H), 2.09-2.12(m, 2H), 2.18-2.19(m, 1H), 2.40-2.42(m, 2H), 2.49-250 (m, 1H), 2.64-2.66(m, 2H), 2.71-2.73(m, 2H), 2.73-2.85(m, 1H), 3.37-3.45(m, 4H), 3.68-3.70(m, 8H) , 3.73-3.76(m, 8H), 5.09-5.71(m, 2H), 6.65-6.71(m, 4h), 6.96-7.00(m, 1H), 7.02-7.08(m, 2H), 7.12-7.22( m, 7H), 7.30-7.31 (m, 2H), 7.37-7.39 (m, 2H). MS (ESI): 839.7 (C 46 h 52 Cl 4 N 2 o 4 )[M+H] + , and the calculated M / z was 838.6. Structural chara...
Embodiment 2
[0041] Example 2: In vitro targeted release pharmacokinetics of HN-CBL in tumor cells
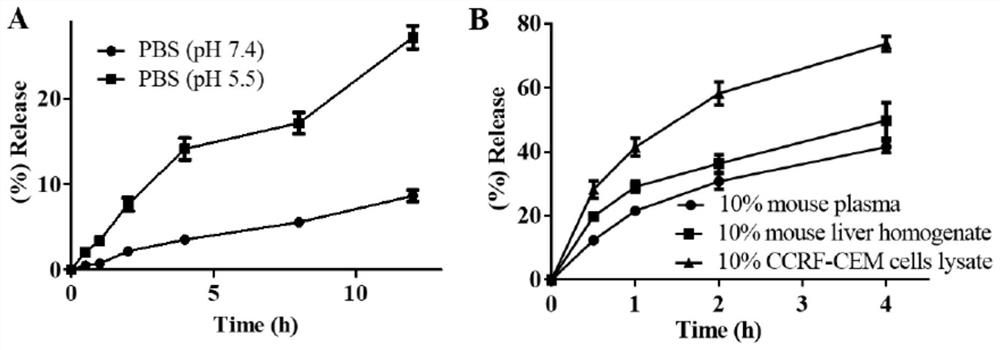
[0042] According to the characteristics of prodrug HN-CBL and carbonate ester cleavage under the catalysis of intracellular esterase, and then release CBL and HN in biological media such as PBS and plasma, especially in the environment with high esterase content and low pH value Released in tumor tissue or cancer cells. To test the above intuitive hypothesis about the prodrug HN-CBL, the release of HN or CBL in different media was evaluated by HPLC-MS. The hydrolytic release of the prodrug HN-CBL was determined at 37°C (pH = 7.4 or 5.5, 10% fresh plasma and 10% cancer cell lysis) in different biological media such as PBS. The degradation of HN-CBL and the generation of HN or CBL were measured by high-performance liquid chromatography-mass spectrometry (HPLC-MS). from figure 1 It can be seen from the results that the hydrolysis rate of HN-CBL in normal isotonic buffer PBS of pH=7.4 is <10...
Embodiment 3
[0043] Example 3: HN-CBL selectively inhibits leukemia cell proliferation
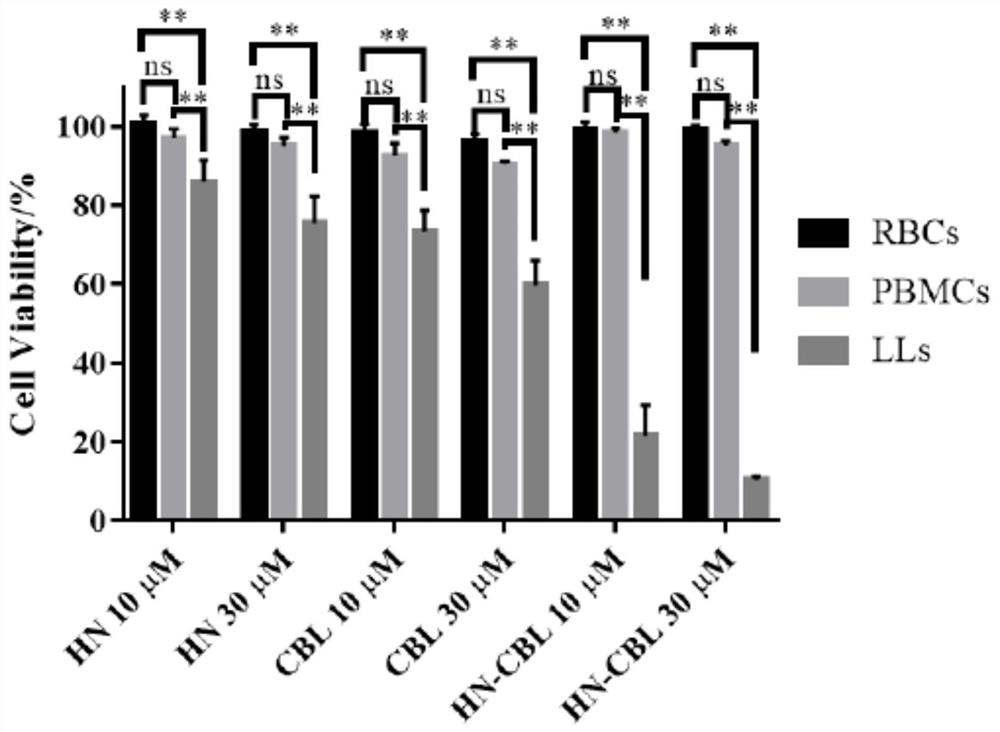
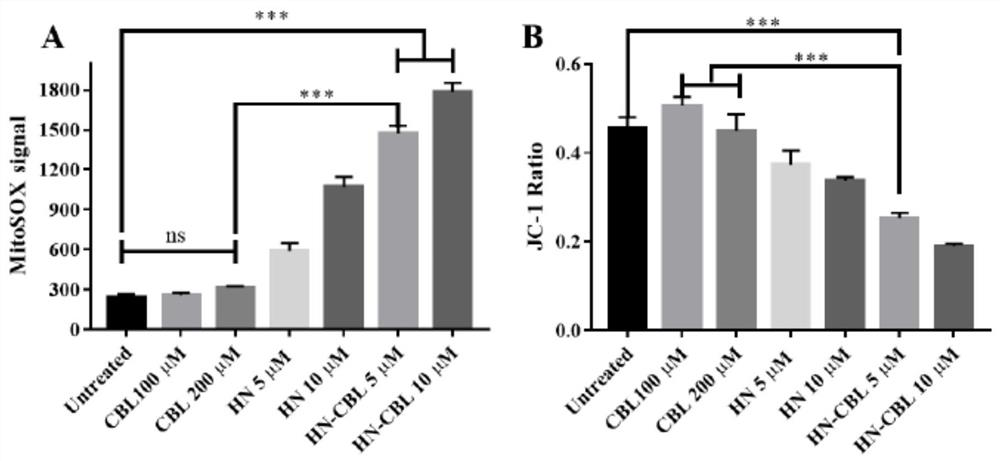
[0044] In this example, the anticancer effect of the prodrug HN-CBL on seven tumor cell lines was studied by MTT colorimetry. Our data show that HN-CBL can effectively reduce the survival rate of seven tested cancer cell lines, namely lymphocytic carcinoma CCRF-CEM (IC50=1.09μM), Jurkat (IC50=1.15μM), U937 (IC50=1.29μM ), MV4-11 (IC50=2.78μM), K562 (IC50=4.86μM), lung cancer A549 (IC50=25.10μM), human liver cancer HepG2 (IC50=24.50μM), there was no significant effect on two normal cells LO2 and NIH3T3 Cytotoxicity. These results indicated that HN-CBL has a broad anti-tumor spectrum, especially selective for leukemia cells. Among the seven human cancer cell lines tested, the IC50 values of HN-CBL were lower than those of CBL and HN, indicating that the synergistic antitumor activity of HN-CBL was stronger than that of HN and CBL (Table 1).
[0045] Table 1 IC50 of HN, CBL and HN-CBL on tumor cell l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com