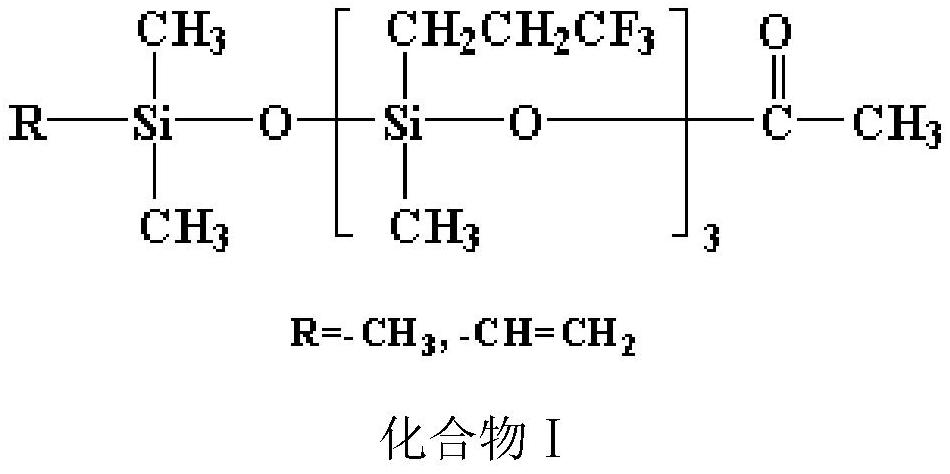
A kind of neutralizing agent for synthesizing polyfluorosiloxane and its preparation method and application
A technology of polyfluorosiloxane and trifluoropropylmethylcyclotrisiloxane, which can be used in chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, organic chemistry, etc., and can solve poor compatibility. , neutralize the problems of inhomogeneity and deterioration, and achieve the effect of consistent reactivity, low production cost and convenient process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method for the neutralizing agent of synthetic polyfluorosiloxane, comprises steps as follows:
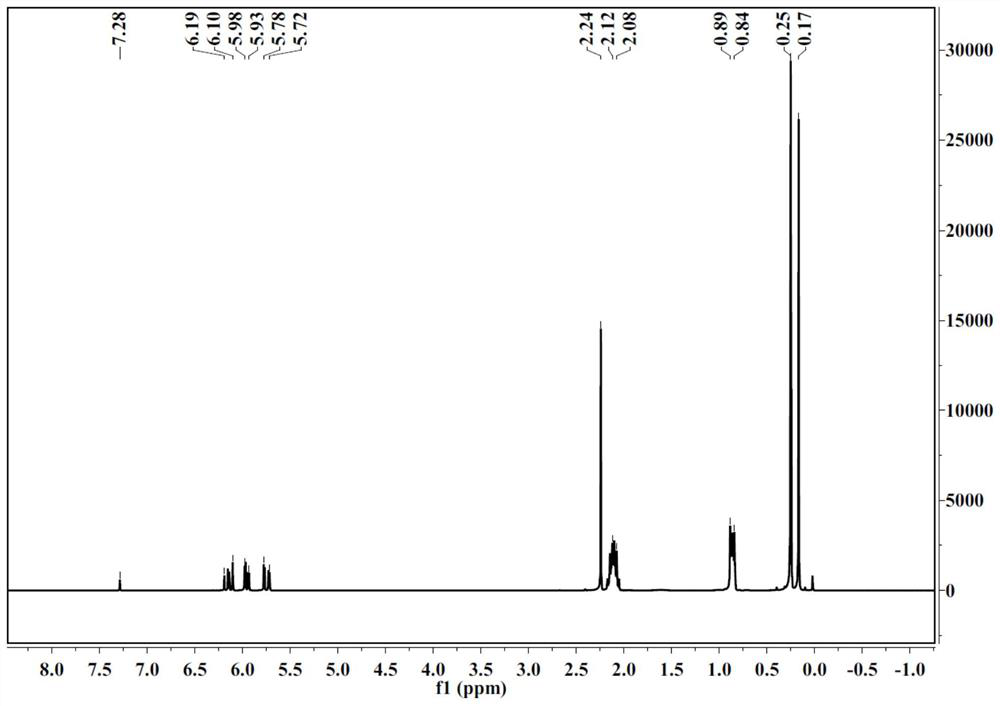
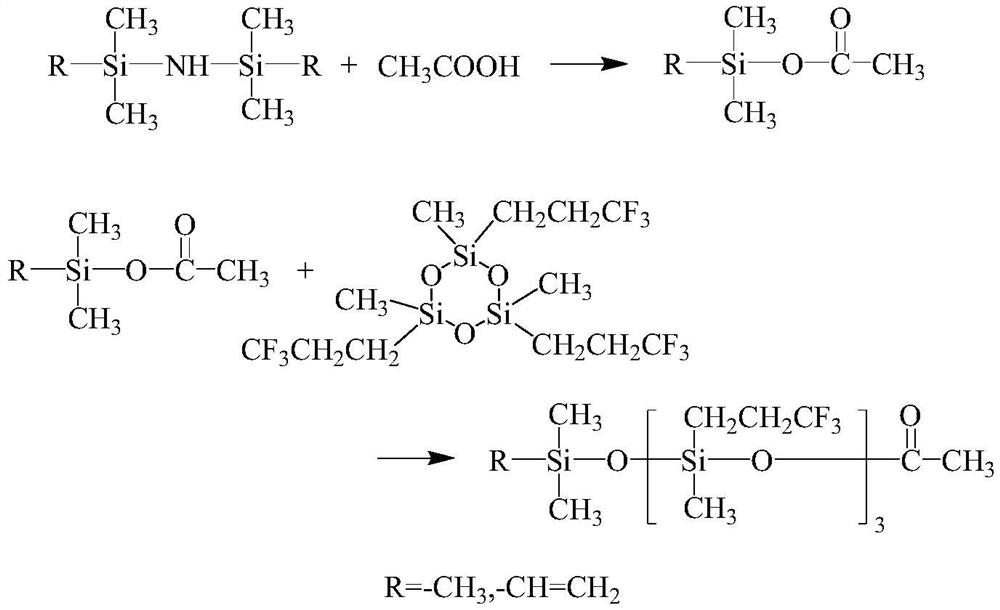
[0034] (1) Add 370g of divinyltetramethyldisilazane to a reactor equipped with a stirring and dropping device, add 260g of acetic acid dropwise, maintain the reaction temperature in a water bath at 40-50°C, and add the dropwise time for 2 hours, After the dropwise addition, keep the system temperature at 40-50°C and continue to react for 1 hour, filter, and rectify the filtrate under atmospheric pressure under the protection of nitrogen. dimethylacetoxysilane;
[0035] (2) Add 216g vinyldimethylacetoxysilane and 468g D 3 F, cool down to 5-15°C, add 3g trifluoromethanesulfonic acid, react for 1.5h, add 2g sodium acetate to neutralize the catalyst after the reaction, stir for 0.5h, filter the reaction solution after returning to room temperature, and decompress the filtrate under 266Pa vacuum Rectify, according to the reflux ratio of 8:1, collect the fr...
Embodiment 2
[0039] A kind of preparation method for the neutralizing agent of synthetic polyfluorosiloxane, comprises steps as follows:
[0040] (1) Add 322g of hexamethyldisilazane to the reactor with a stirring and dropping device, add dropwise 250g of acetic acid, maintain the reaction temperature in a water bath at 40-50°C, add the time for 2 hours, and complete the dropping Afterwards, keep the system temperature at 40-50°C and continue to react for 1h, filter, and the filtrate is rectified under normal pressure under the protection of argon. Oxysilane;
[0041] (2) Add 198g trimethylacetoxysilane and 468g D 3 F, lower the temperature to 5-15°C, add 3g of trifluoromethanesulfonic acid, react for 2h, add 2g of sodium acetate to neutralize the catalyst after the reaction, stir for 0.5h, filter the reaction solution after returning to room temperature, and filter the filtrate under vacuum at 266Pa Distillation, according to the reflux ratio of 8:1 to collect the 92 ~ 95 ° C fraction, ...
Embodiment 3
[0044] A kind of preparation method for the neutralizing agent of synthetic polyfluorosiloxane, comprises steps as follows:
[0045] (1) Add 370g of divinyltetramethyldisilazane to a reactor equipped with a stirring and dropping device, add 245g of acetic acid dropwise, maintain the reaction temperature in a water bath at 40-50°C, and add the dropwise time for 1h, After the dropwise addition, keep the system temperature at 40-50°C and continue to react for 1 hour, filter, and rectify the filtrate under atmospheric pressure under the protection of nitrogen. Dimethylacetoxysilane;
[0046] (2) Add 375g vinyl dimethyl acetoxy silane and 468g D 3 F, lower the temperature to 5-15°C, add 2g trifluoromethanesulfonic acid, react for 1.5h, add 1.5g sodium acetate to neutralize the catalyst after the reaction, stir for 0.5h, filter the reaction solution after returning to room temperature, and reduce the filtrate under vacuum at 266Pa Pressure rectification, according to the reflux ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com