Warfarin medication gene detection kit and use method thereof
A gene detection and kit technology, applied in the field of warfarin drug gene detection kits, can solve the problems of high sample processing requirements, low specificity, long detection cycle, etc., and achieves short detection cycle, high sensitivity, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Primer and probe combination design and use
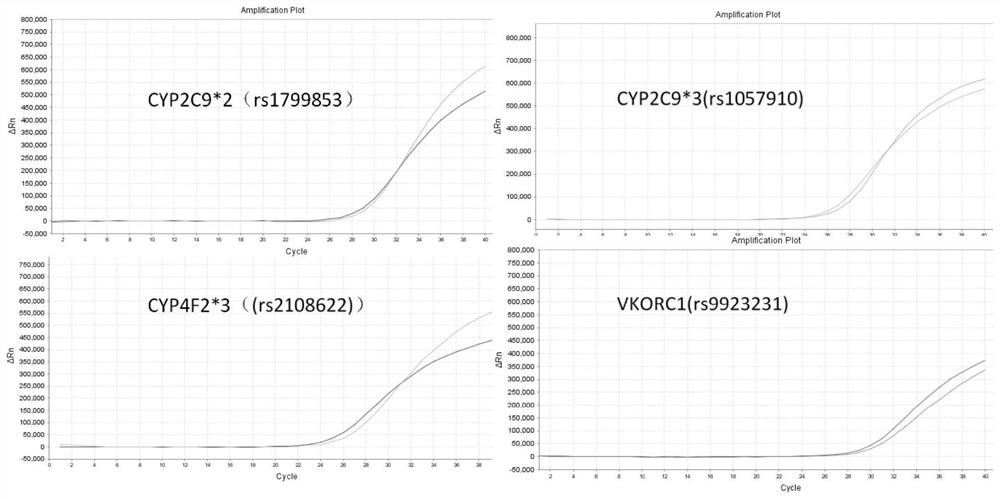
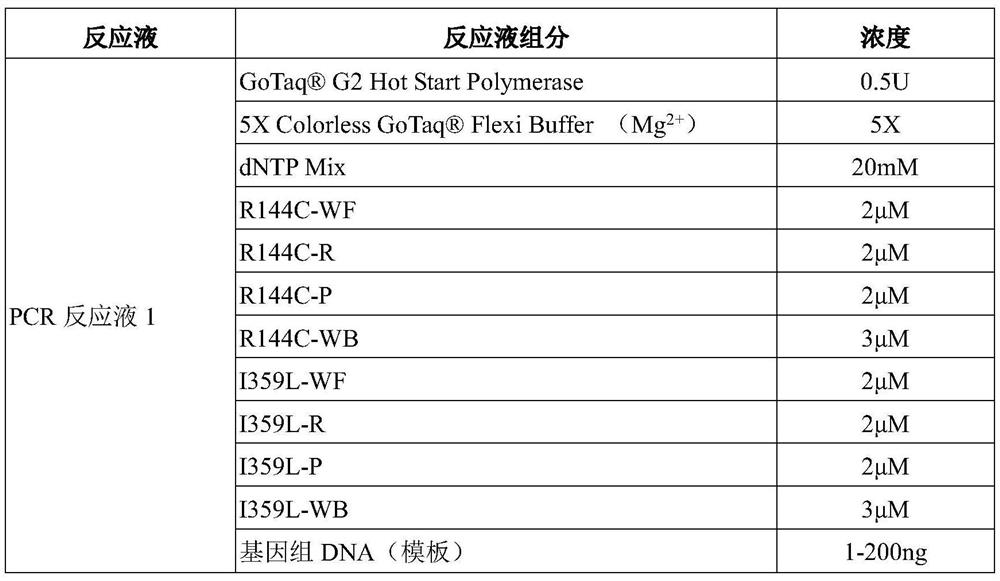
[0041] A warfarin medication gene detection kit, including primer pairs, probes and inhibitors for detecting CYP2C9*2, CYP2C9*3, CYP4F2*3 and VKORC1 gene polymorphisms to be detected, PCR reaction solution, Positive quality control and negative quality control; the sites to be tested are rs1799853 site of CYP2C9*2(430C>T) gene, rs1057910 site of CYP2C9*3(1075A>C) gene, CYP4F2*3(1297G>A At least one of the rs2108622 site of the ) gene and the rs9923231 site of the VKORC1 (-1639G>A) gene. The present invention is screened through large-scale experiments, and the preferred primer and probe sequences are as follows:
[0042] 1. Primer and probe combination for identifying CYP2C9*2(430>C):
[0043] R144C-WF: GGAAGAGGAGCATTGAGGGAC (SEQ ID NO. 1)
[0044] R144C-R:ACAACCAGGACTCATAATGAAAG (SEQ ID NO.3)
[0045] R144C-P: FAM-AAAACCAAGGGTGGGTGACCCTACTCCA-TRAMA (SEQ ID NO. 4)
[0046] R144C-WB: GGATGGGGAAGAGGAGCATTGAGGACTG...
Embodiment 2
[0088] Embodiment 2: the preparation method of kit
[0089] 1. Acquisition of positive quality control products
[0090] The method of obtaining the positive quality control product is as follows: according to the CYP2C9*2, CYP2C9*3, CYP4F2*3 and VKORC1 gene sequences published by the NCBI database, construct a synthetic sequence gene fragment of the plasmid, and then insert the fragment into the T vector, using Escherichia coli The DH5α strain was transformed and the plasmid was extracted, and the quality control plasmids were mixed in equal proportions to be the positive quality control product.
[0091] 2. Preparation of negative quality control
[0092] The negative quality control substance adopted in the present invention is DEPC-treated deionized water.
[0093] 3. Configuration of PCR reaction system
[0094]The kit of the present invention is designed with 8 PCR reaction strips, and each reaction strip can complete the detection of 2 people. Tubes 1-4 are composed ...
Embodiment 3
[0102] Embodiment 3: the use of gene detection kit
[0103] The method for using the kit of the present invention will be described in detail below in conjunction with specific drawings.
[0104] 1. Sample collection
[0105] In this embodiment, peripheral blood of 10 patients was collected, numbered 1-10 respectively. Use vacuum blood collection tubes containing EDTA anticoagulant to collect 5ml of blood samples, let stand at room temperature for 30 minutes, centrifuge at 1500-2000rpm for 10 minutes, and collect plasma and blood cells in sterile screw-top plastic tubes.
[0106] 2. Sample nucleic acid extraction
[0107] Nucleic acid extraction was performed on the vacuum blood sample collected in step 1, and a commercial kit was used for nucleic acid extraction; this invention uses the HiPure Blood DNA Mini Kit (article number: D3111) produced by Meiji Biotechnology, and the experimental steps are carried out with reference to the following instructions.
[0108] In a 1.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com