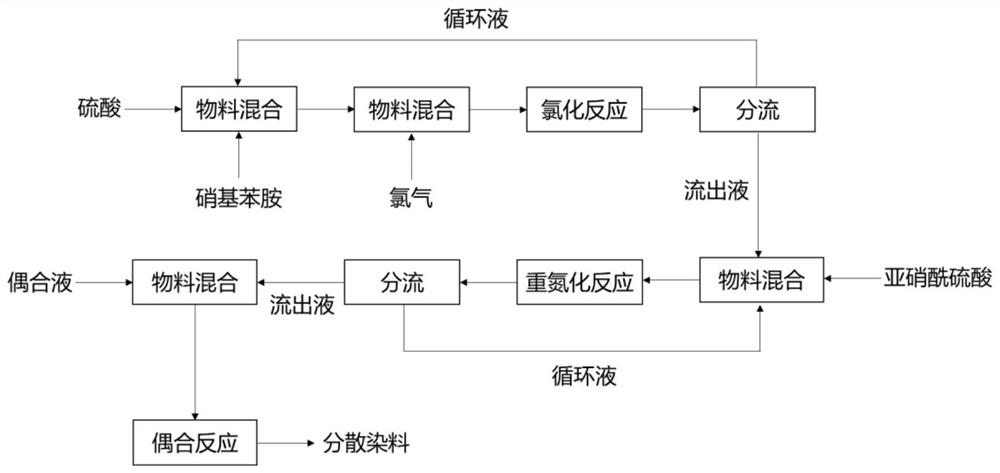
A kind of continuous preparation method of disperse dye
A disperse dye and diazotization technology, which is applied in the field of continuous preparation of disperse dyes, can solve the problems of increasing energy consumption and material consumption in the production process, increasing production costs, and not involving the continuous system construction of downstream processes, so as to improve product quality and Effects of stability, efficiency improvement, and reaction equivalent reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
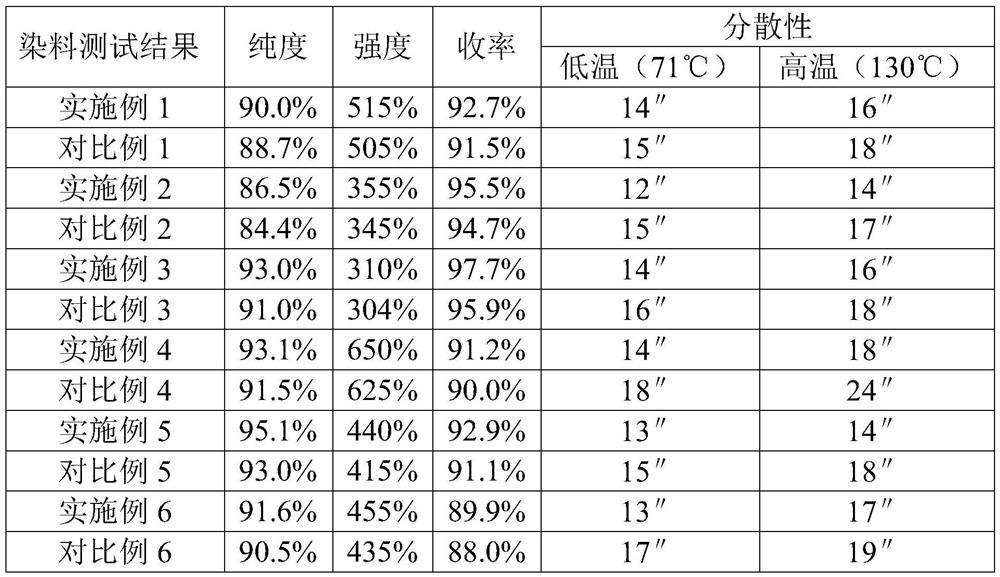
Examples
Embodiment 1
[0032] Example 1 Preparation of Disperse Violet 93:1 by Chlorination and Coupling of 2,4-Dinitroaniline
[0033] 2,4-dinitroaniline is dissolved in 98wt% sulfuric acid, and the mass ratio of 2,4-dinitroaniline to 98wt% sulfuric acid is 1:4; the solution and chlorine are continuously fed into the chlorination mixer, and then enter The chlorination reaction was carried out in a chlorination tubular reactor to obtain a 2,4-dinitro-6-chloroaniline solution; the molar ratio of 2,4-dinitroaniline to chlorine was 1:0.60, and the mixing temperature was 48°C. The temperature is controlled at 50°C, and the reaction time is 2.5 hours; 87.5% of the reaction liquid is circulated back to the chlorination mixing stage, and the remaining part is mixed with nitrosyl sulfuric acid in the diazo mixer and then sent to the diazo tubular reactor. Carry out diazotization reaction to obtain diazo solution, 85% of the diazo solution is circulated back to the diazo mixing stage, and the rest is input i...
Embodiment 2
[0035] Example 2 Preparation of Dispersed Ruby 167 by Chlorination and Coupling of p-Nitroaniline
[0036] p-nitroaniline is dissolved in 95wt% sulfuric acid, and the mass ratio of p-nitroaniline and 95wt% sulfuric acid is 1:2.5; O-chloro-p-nitroaniline solution was obtained by chemical reaction; the molar ratio of p-nitroaniline to chlorine gas was 1:0.55, the mixing temperature was 29°C, the reaction temperature was controlled at 30°C, and the reaction time was 2.5h; 89% of the reaction solution was circulated Reflux to the chlorination mixing stage, the remaining part is mixed with nitrosyl sulfuric acid in the diazo mixer and then transported to the diazo tank reactor for diazotization reaction to obtain diazonium liquid, and 86% of the diazo liquid is circulated and refluxed At the diazo mixing stage, the remaining part is input into the diazo liquid buffer tank, and the temperature is adjusted to 8°C; the molar ratio of p-nitroaniline to nitrosyl sulfuric acid is 1:1.05,...
Embodiment 3
[0038] Embodiment 3 p-Nitroaniline Chlorination Coupling Disperse Orange 30
[0039]p-Nitroaniline is dissolved in 98wt% sulfuric acid, and the mass ratio of p-nitroaniline to 98wt% sulfuric acid is 1:3; the solution and chlorine gas are continuously fed into the chlorination mixer, and enter the chlorination tubular reactor for chlorination The reaction obtained 2,6-dichloro-4-nitroaniline solution; the molar ratio of p-nitroaniline to chlorine gas was 1:1.10, the mixing temperature was 78°C, the reaction temperature was controlled at 80°C, and the reaction time was 4.5h; 92 % of the reaction solution is circulated back to the chlorination mixing stage, and the remaining part is mixed with nitrosyl sulfuric acid in the diazo mixer and then sent to the diazo tube reactor for diazotization reaction to obtain the diazo solution. 89% The diazo solution is circulated back to the diazo mixing stage, and the remaining effluent is input into the diazo solution buffer tank, and the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com