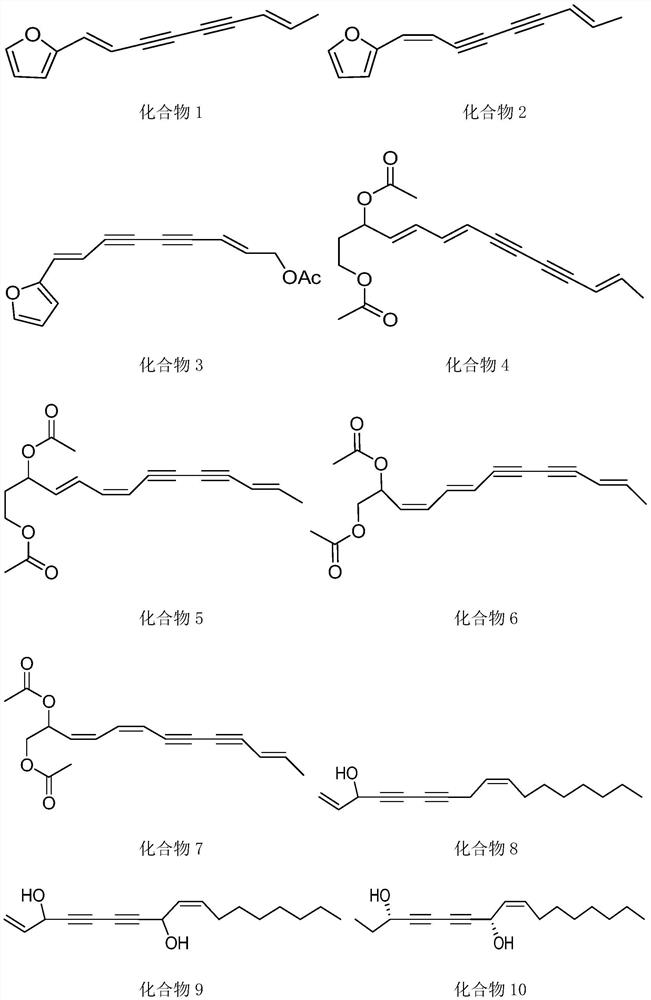
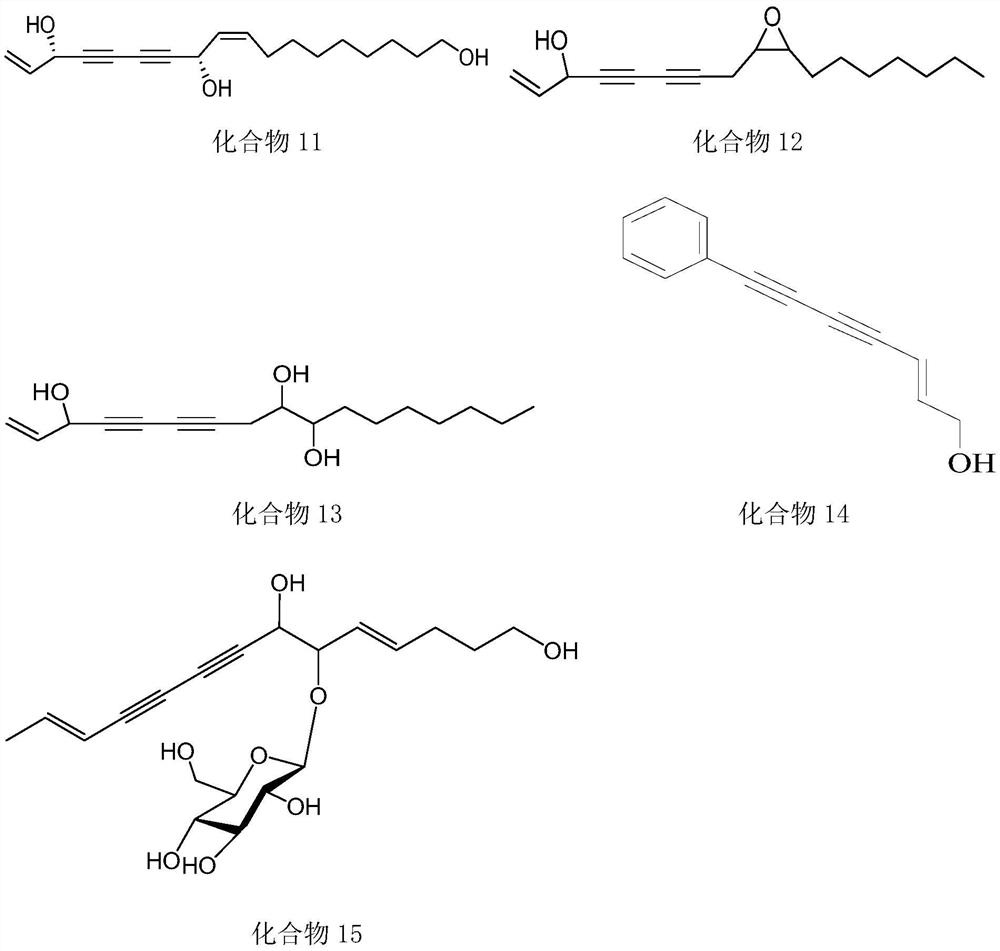
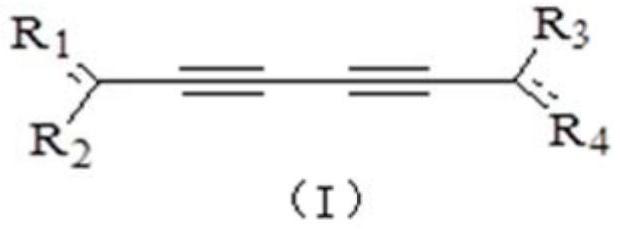New application of polyacetylenes
A compound and polyalkyne technology, applied in the field of chemical medicine, can solve the problems of less content, less research on the relationship between polyalkynes, and insufficient stability, and achieve the effects of reducing toxic side effects, improving safety, and improving uric acid-lowering effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example provides the extraction method and characterization of compounds 4-7 in the active ingredients.
[0035] The ethanol, ethyl acetate, petroleum ether and methanol used in this example are all commercially available products.
[0036]Take 100kg of Atractylodes atractylodis from Compositae, crush them, soak and extract with 15 times the volume of 70% ethanol aqueous solution, the extraction temperature is 50-100°C, the extraction time is 120min, and concentrate under reduced pressure until there is no solvent to obtain a concentrated solution A; concentrated solution A is separated through low-pressure D101 column (column diameter 28cm * high 162cm, column volume 100L), utilizes 30%, 95% ethanol-water eluting 4BV respectively, collects 95% position; After this position is concentrated (solid content About 3kg) was separated by LX-20SS column (column diameter 20cm×height 78cm, column volume 25L), eluted 3BV with 70%, 80% ethanol-water respectively, then eluted ...
Embodiment 2
[0048] This example provides the extraction method and characterization of compounds 8-11 in the active ingredients.
[0049] The n-pentane, ethyl acetate, diethyl ether, ethanol and methanol used in this example are all commercially available products.
[0050] Cut 20kg of carrots into small pieces, chop them with a mixer, and then use 40L of n-pentane and 30L of ethyl acetate to extract 3 times at room temperature successively, collect the n-pentane extract and the ethyl acetate extract respectively, and reduce Concentrate under reduced pressure to remove the organic solvent to obtain 20 g of n-pentane extract (ie: component A) and 15 g of ethyl acetate extract (ie: component B) respectively. Component B was dissolved in 35 mL of a mixed solution of n-hexane and ethyl acetate, wherein the volume ratio of n-hexane and ethyl acetate was 95:5, and passed through a silica gel column (column diameter 6 cm × height 60 cm, column volume 1.7 L) ) separation and purification, using ...
Embodiment 3
[0058] This example provides the extraction method and characterization of compounds 12-13 in the active ingredients.
[0059] The ethanol, n-hexane, chloroform, ethyl acetate and methanol used in this example are all commercially available products.
[0060] 20 kg of medicinal ginseng was crushed, then soaked and extracted with 15 times the volume of 80% ethanol aqueous solution at room temperature for 1 week, extracted twice, combined the extracts, concentrated under reduced pressure to remove the organic solvent, and obtained a concentrate. The concentrate was extracted with 1 volume of n-hexane for three times, the n-hexane layers were combined, concentrated under reduced pressure to remove the organic solvent, and the n-hexane extract was obtained. The n-hexane extract was separated by ODS reverse-phase silica gel column chromatography (column diameter 6cm×height 60cm, column volume 1.7L), and the mixed solution of n-hexane and ethyl acetate was used as the mobile phase t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com