A metallo-beta-lactamase inhibitor and its preparation method and application
A lactamase and inhibitor technology, applied in the field of new antibacterial drug development, can solve problems such as inability to effectively eliminate metallo-beta-lactamase drug resistance, and achieve the effects of easy industrial production, high inhibition efficiency and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
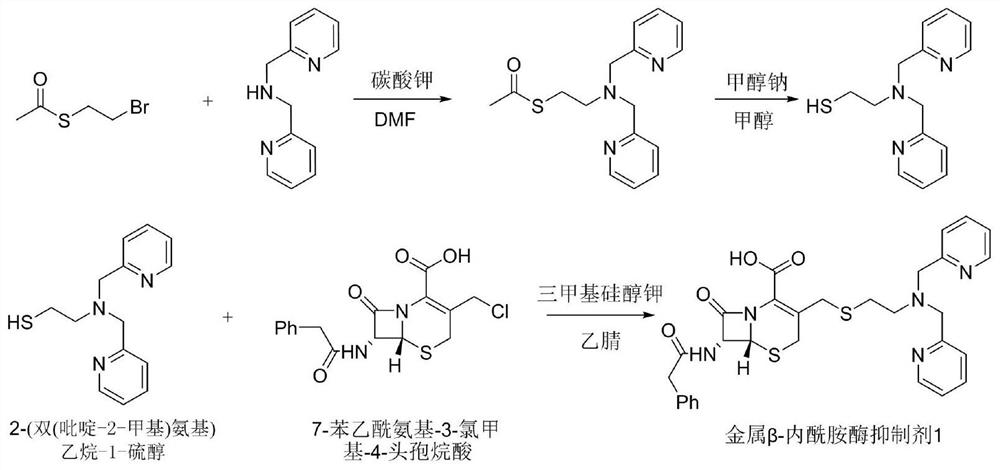
[0049] Synthetic route such as figure 1 As shown, it specifically includes the following steps:
[0050] (1) Dissolve lutinamine (200mg, 1.0mmol) in DMF (4mL), then add S-(2-bromoethyl) ester (220mg, 1.2mmol) and potassium carbonate (1.5mmol), 80 Stir at ℃, react for 12 hours, stop heating, cool to room temperature, pour the reaction product into a separatory funnel, add 15 mL of dichloromethane and water (3ⅹ10 mL) for extraction, collect the organic phase; add anhydrous sodium sulfate to the organic phase Dry and distill under reduced pressure to obtain the crude product. The crude product was purified with a silica gel column; wherein, methanol: dichloromethane = 5% to 15%, and the mercapto-protected product was obtained as a brown oily liquid (225mg), and the yield was 75%;
[0051] (2) Dissolve the thiol-protected product (225 mg, 0.75 mmol) in methanol (5 mL), add sodium methoxide (81 mg, 1.5 mmol), stir at room temperature, react for 1.5 hours, and pour the reaction pro...
Embodiment 2
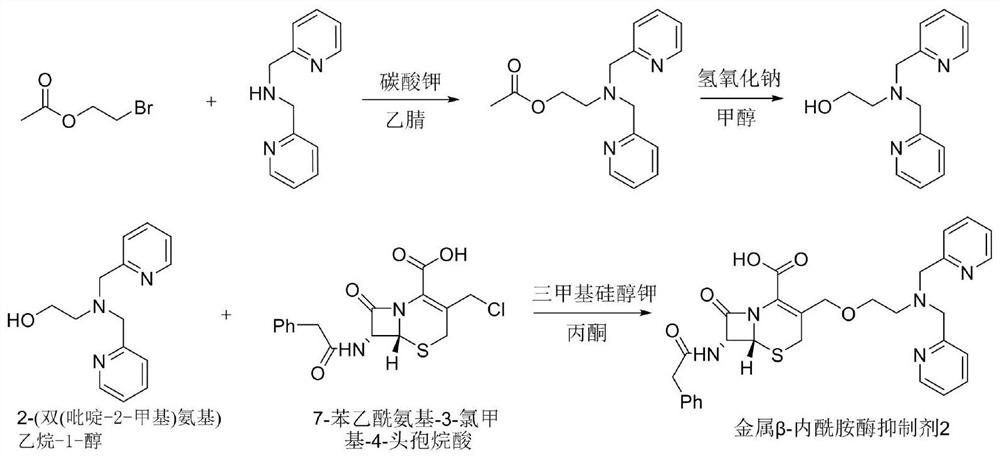
[0054] Synthetic route such as figure 2 As shown, it specifically includes the following steps:
[0055] (1) Dissolve lutinamine (200mg, 1.0mmol) in acetonitrile (6mL), then add ethyl 2-bromoacetate (250mg, 1.5mmol) and potassium carbonate (2.5mmol), stir at 100°C, React for 11 hours, stop heating, cool to room temperature, pour the reaction product into a separatory funnel, add 15 mL of dichloromethane and water (3ⅹ10 mL) for extraction, collect the organic phase; add anhydrous sodium sulfate to the organic phase to dry and reduce pressure The crude product was obtained by distillation, and the crude product was purified with a silica gel column; wherein, methanol: dichloromethane = 5% to 15%, and the hydroxyl-protected product was obtained as a yellow oily liquid (194mg), and the yield was 68.3%;
[0056] (2) Dissolve the hydroxyl-protected product (194 mg, 0.68 mmol) in methanol (8 mL), add sodium hydroxide (1.2 mmol), stir at room temperature, react for 2.5 hours, and po...
Embodiment 3
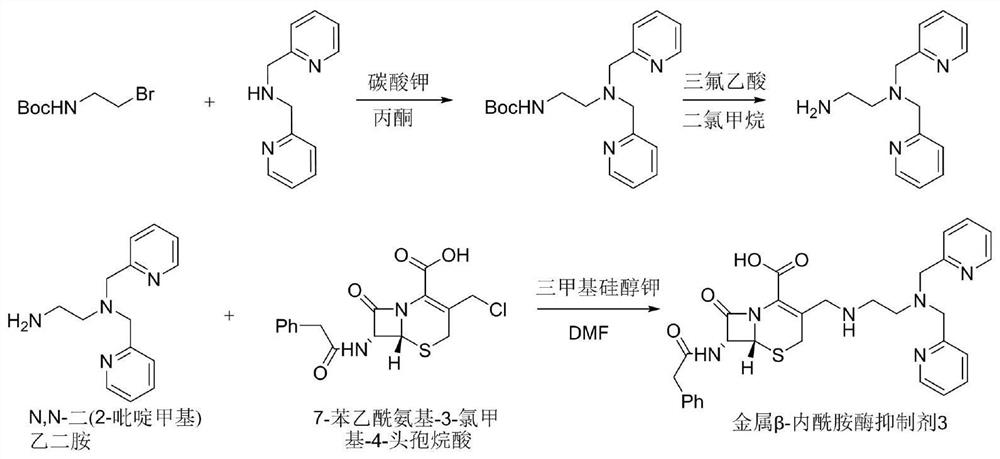
[0059] Synthetic route such as image 3 As shown, it specifically includes the following steps:
[0060] (1) Dissolve lutinamine (200mg, 1.0mmol) in acetone (5mL), then add (2-bromoethyl) tert-butyl carbamate (269mg, 1.2mmol) and potassium carbonate (280mg, 2.0 mmol), stirred at 80°C, reacted for 13 hours, stopped heating, cooled to room temperature, poured the reaction product into a separatory funnel, added 15 mL of dichloromethane and water (3ⅹ10 mL) for extraction, collected the organic phase; added to the organic phase Drying over anhydrous sodium sulfate and distilling under reduced pressure to obtain the crude product. The crude product was purified with a silica gel column; wherein, methanol:dichloromethane=5% to 15%, the product protected by the amino group was obtained as a brown oily liquid (317mg), and the yield was 92.8%;
[0061] (2) Dissolve the amino-protected product (317mg, 0.92mmol) in dichloromethane (8mL), add trifluoroacetic acid (2mL), stir at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com