Uses of flavonoids of marine fungal origin
A technology of flavonoids and marine fungi, applied in the field of medical applications, to achieve the effects of protecting oxidative stress, reducing the expression of ROS, and protecting against
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of benzo-γ-pyrone and 2,3,4,6,8-pentahydroxyl-1-methylflavone (preparation method quoted from "New Chromones from a Marine-Derived Fungus, Arthrinium sp ., and Their Biological Activity", Jie Bao et al., Molecules 2018,23,1982. 2,3,4,6,8-pentahydroxy-1-methylflavone is numbered 73, and benzo-γ-pyrone is numbered for 75)
[0056] The specific operation is as follows:
[0057] (1) Inoculate the metabolites of the marine fungus ArthriniμM sp.UJNMF0008 on the plate, put the inoculated bacteria in the culture medium, shake the bacteria in a shaker at 28°C and 160r for 3 days to obtain the seed liquid of UJNMF008, and inoculate UJNMF0008 The seed liquid was transferred to a 1 L Erlenmeyer flask with solid rice medium. Cultured statically at 28°C for 30 days. After fermentation, it was extracted three times with 95% ethanol. The EtOH extract was evaporated under reduced pressure to obtain an aqueous solution, then extracted three times with ethyl ace...
Embodiment 2
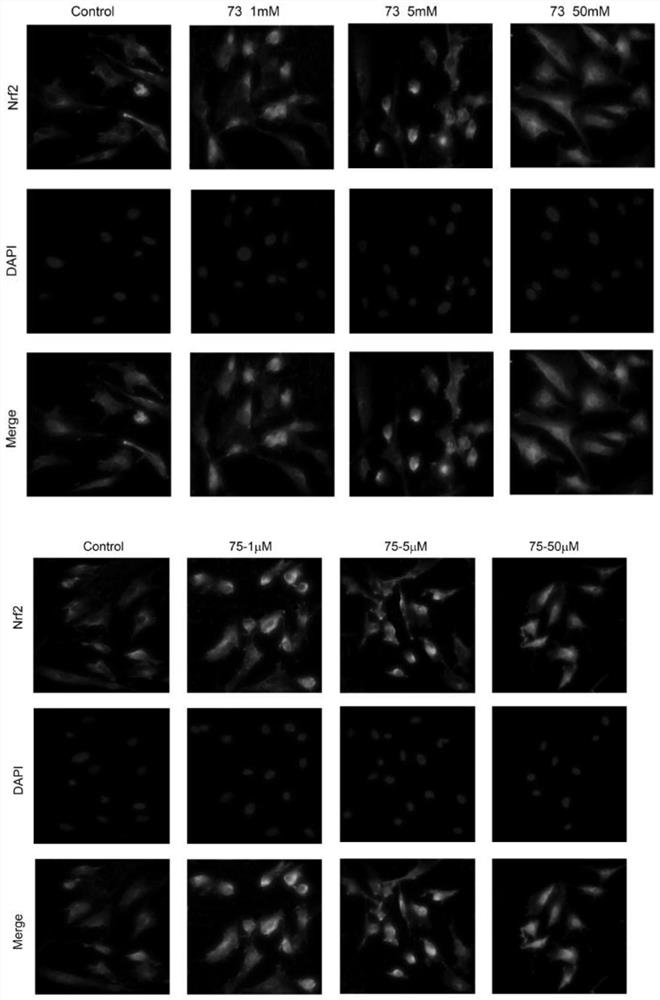
[0060] Example 2: Benzo-γ-pyrone and 2,3,4,6,8-pentahydroxy-1-methylflavone can promote Nrf2 into the nucleus
[0061]Experimental method: inoculate endothelial cells (the endothelial cells used in the present invention are obtained from the umbilical cord of the puerpera from the 456 Hospital of the People's Liberation Army, take out and store in low temperature, and extract within 2 hours) in a 2ml culture dish. After the cells are spread to 80%, the normal group: Add LDL culture under normal conditions without serum, set up control group: culture under normal conditions without serum; experimental group: add benzo-γ-pyrone and concentrations of 1 μM, 5 μM and 50 μM under serum-free conditions, respectively 1μM, 5μM and 50μM 2,3,4,6,8-pentahydroxy-1-methylflavone were incubated for 24 hours and then treated with drugs. 37°C, CO 2 After culturing in the incubator for 6 hours, discard the old culture medium, gently wash the cells twice with 0.1M PBS; discard the 0.1M PBS, fix...
Embodiment 3
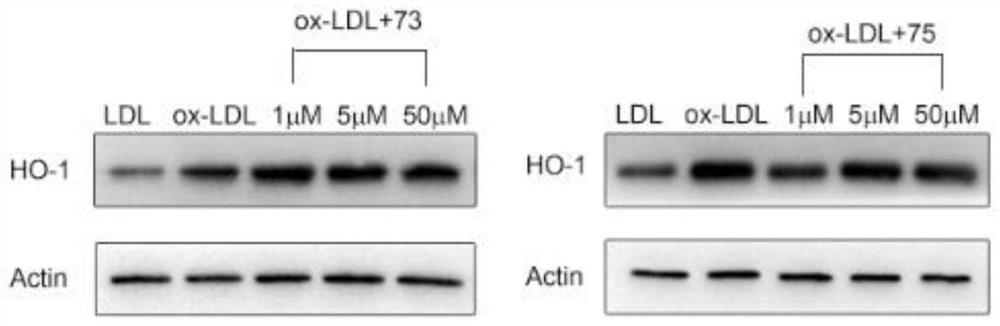
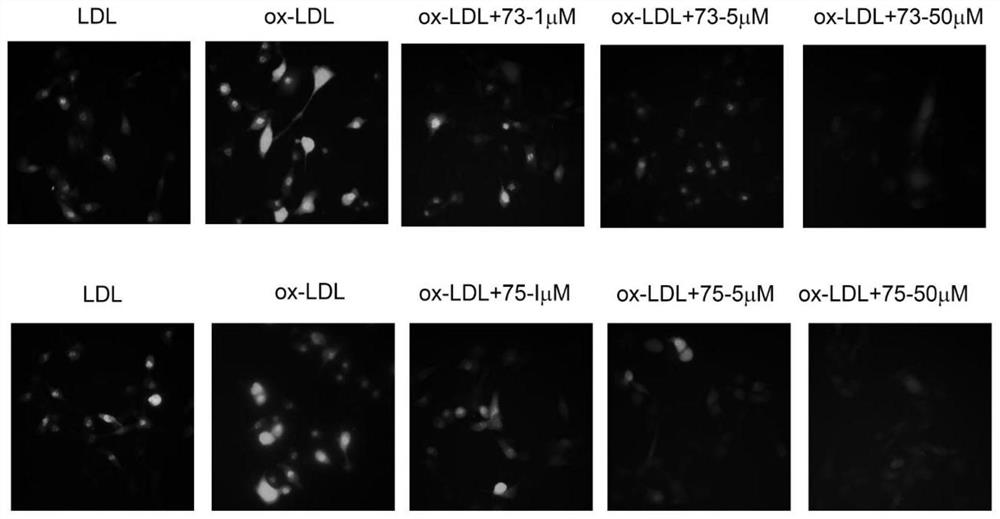
[0062] Example 3: Benzo-γ-pyrone and 2,3,4,6,8-pentahydroxy-1-methylflavone can activate nrf2 downstream antioxidant protein HO-1
[0063] Experimental method: Inoculate endothelial cells in a culture dish with a diameter of 6 cm. Normal group: add LDL culture under normal conditions without serum, set up control group: add ox-LDL culture under normal conditions without serum; Add 2,3,4,6,8-pentahydroxy-1-methylflavone at concentrations of 1 μM, 5 μM and 50 μM, and 1 μM, 5 μM and 50 μM benzo-γ-pyrone under the conditions for incubation. 37°C, CO 2 After culturing in the incubator for 6 hours, the cells were lysed, the cell lysate was collected, centrifuged at 12000 g at 4 °C for 15 min, the supernatant was taken, and the expression level of HO-1 in endothelial cells was detected by western blot.
[0064] The results showed that: compared with the ox-LDL group in the control group, the addition of compound 2,3,4,6,8-pentahydroxy-1-methylflavone (1 μM, 5 μM, 50 μM) while adding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com