Arylamine compound and organic electroluminescent device thereof
A compound and aromatic amine technology, applied in the field of aromatic amine compounds and organic electroluminescent devices, can solve the problems of reduced luminous efficiency of devices, unbalanced charge of light-emitting layer, shortened service life, etc., and achieves improved refractive index and aging rate. Low, prolonged service life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Preparation of intermediate M1:
[0074]
[0075] Compound A1 and compound Y1 undergo a C-N coupling reaction under the conditions of base, catalyst and ligand to obtain intermediate M1;
[0076] Preparation of intermediate M2:
[0077]
[0078] Compound A2 and compound Y2 undergo a C-N coupling reaction under the conditions of base, catalyst and ligand to obtain intermediate M2;
[0079] The preparation of arylamine compound shown in general formula (I):
[0080]
[0081] The intermediate M1 and the intermediate M2 undergo a C-N coupling reaction under conditions such as a base and a catalyst to obtain an arylamine compound represented by the general formula (I).
[0082] Wherein, said X is selected from O or S,
[0083] The L is selected from one of a single bond, a substituted or unsubstituted arylene group with 6 to 18 carbon atoms, a substituted or unsubstituted heteroarylene group with 3 to 12 carbon atoms,
[0084] The L 1 , L 2 one independently sel...
Synthetic example 1
[0104] Synthesis Example 1: Synthesis of Compound 1
[0105]
[0106] Synthesis of intermediate M1-1: under an argon atmosphere, successively add Y1-1 (2.95g, 12mmol) and A1-1 (1.69g, 10mmol) and 50ml toluene, then add sodium tert-butoxide (1.44g , 15mmol), Pd 2 (dba) 3 (0.46g, 0.5mmol) and tri-tert-butylphosphine (0.1g, 0.5mmol), stirred and heated to reflux, and reacted for 24 hours; after the reaction, cooled to room temperature, filtered, and the filtrate was distilled under reduced pressure, and then passed through the column layer Purified by analysis to obtain intermediate M1-1 (2.98g, 8.9mmol), with a HPLC purity of 99.4% and a yield of 89%.
[0107] Synthesis of intermediate M2-1: To the system of Y2-1 (3.73g, 10mmol) and A2-1 (2.54g, 15mmol) dissolved in toluene (85ml), add Pd in sequence 2 (dba) 3 (0.28g, 0.3mmol), tri-tert-butylphosphine (0.12g, 0.6mmol) and sodium tert-butoxide (2.88g, 30mmol), heated to 100°C, and reacted for 12 hours; after the reaction...
Synthetic example 2
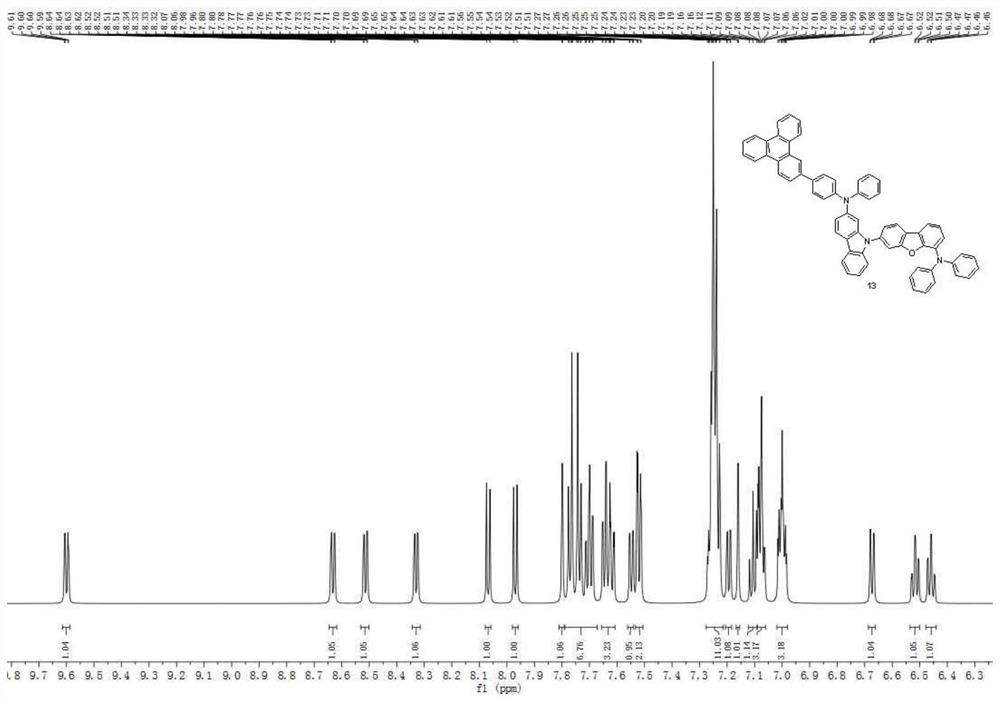
[0109] Synthesis Example 2: Synthesis of Compound 13
[0110]
[0111]
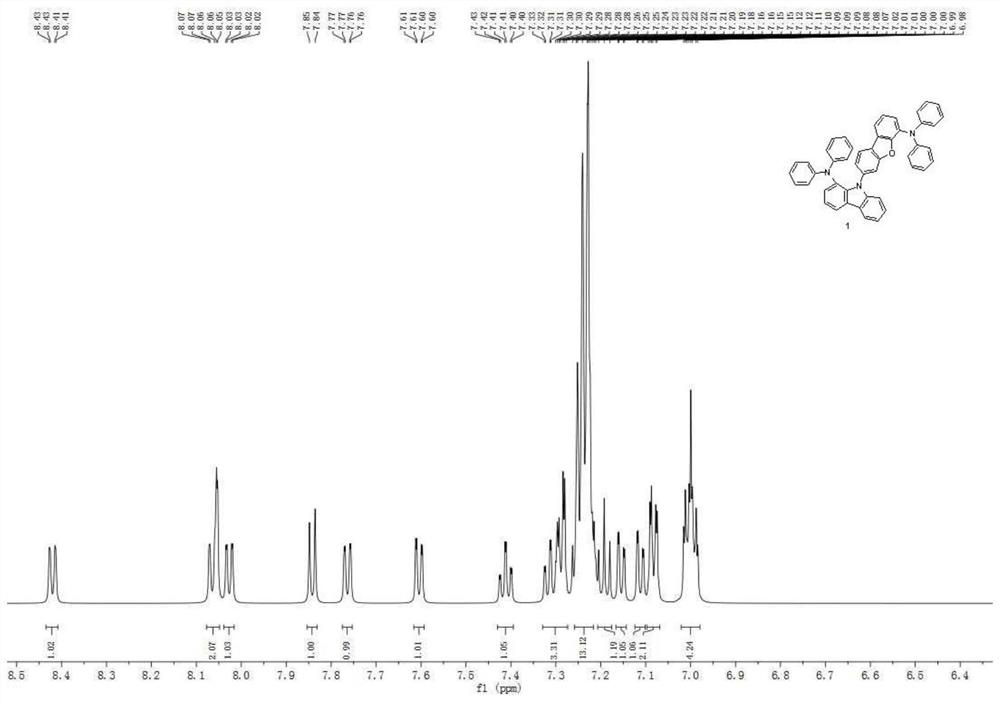
[0112] The raw materials and intermediates in Synthesis Example 1 were converted accordingly, and according to the synthesis method of Compound 1 in Synthesis Example 1, Compound 13 was obtained, with an HPLC purity of 99.5% and a yield of 76%. Mass Spectrum m / z: 895.03 (calculated: 894.09). Theoretical element content (%)C 66 h 43 N 3O: C, 88.66; H, 4.85; N, 4.70; O, 1.79. Actual element content (%): C, 88.59; H, 4.83; N, 4.75; O, 1.85. 1 H NMR (600MHz, CDCl 3 )(δ,ppm):9.60(dd,1H),8.63(dd,1H),8.51(dd,1H),8.33(dd,1H),8.07(d,1H),7.97(d,1H),7.80 (d,1H),7.79-7.67(m,7H),7.66-7.61(m,3H),7.55(dd,1H),7.52(dd,2H),7.28-7.22(m,11H),7.19(dd ,1H),7.16(d,1H),7.11(t,1H),7.10-7.06(m,3H),7.02-6.98(m,3H),6.67(dd,1H),6.52(td,1H), 6.46(td,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com