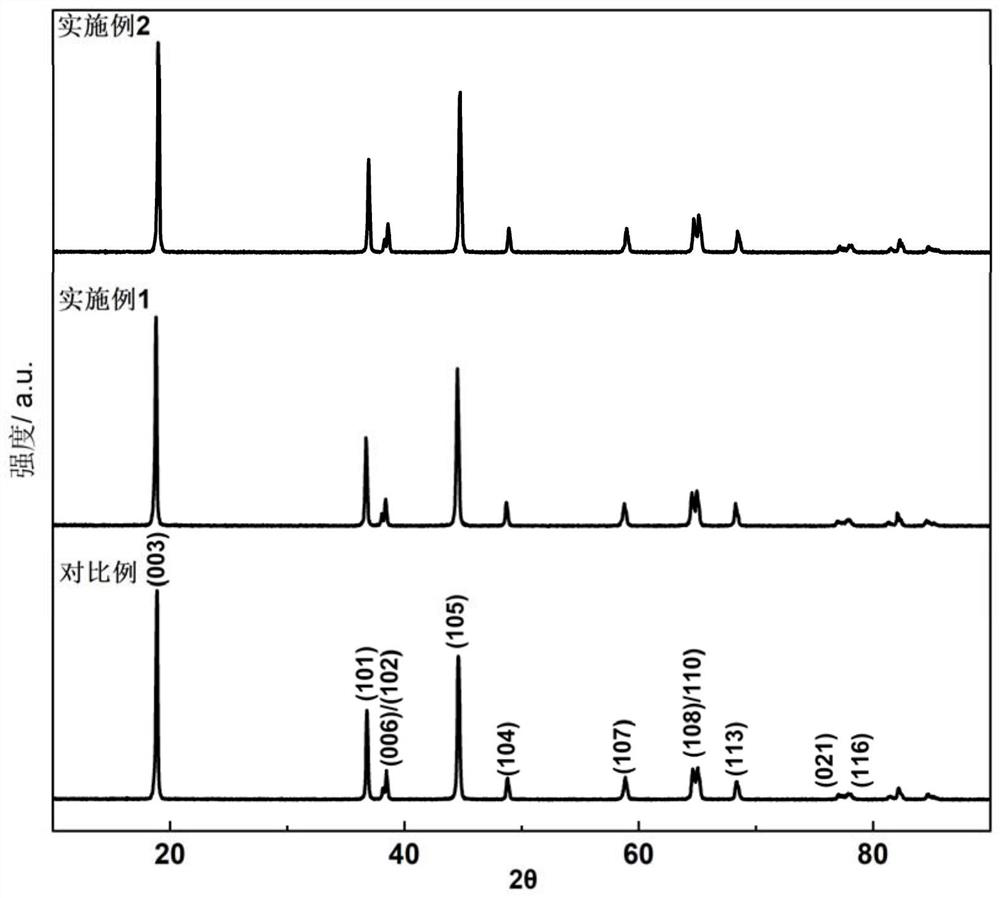
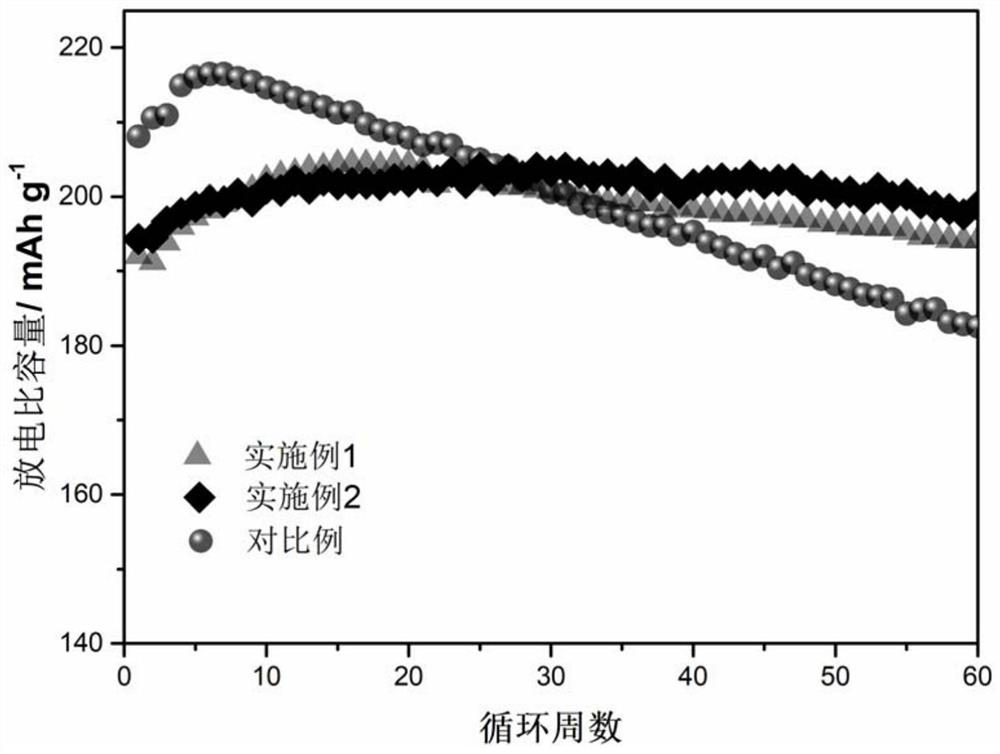
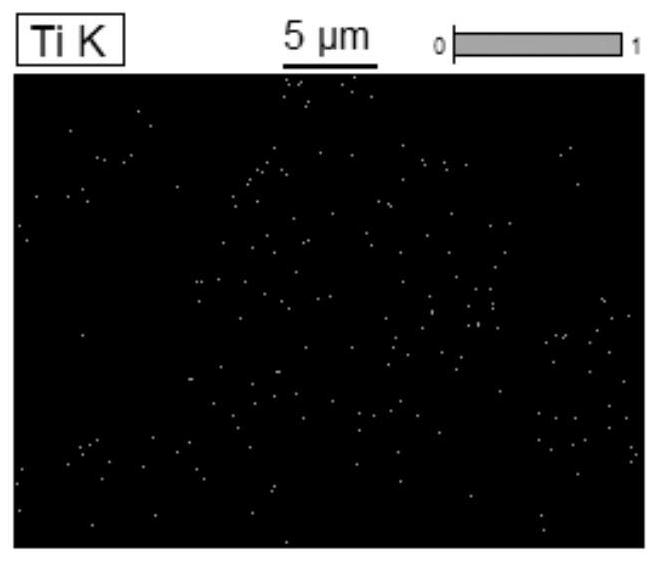
High-nickel positive electrode material with reconstructed primary particle surface layer and preparation method thereof
A cathode material and surface structure technology, applied in the field of high-nickel anode materials and their preparation, can solve problems such as poor cycle stability and structural stability damage of high-nickel anode materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Weigh NiSO in the ratio of Ni:Co=92:8 in molar ratio 4 ·6H 2 O and CoSO 4 ·7H 2 O, the total concentration of Ni and Co ions is 4mol L with deionized water -1 mixed salt solution, and then use deionized water to prepare NaOH and ammonia water in a molar ratio of 3:1 to make the NaOH concentration 0.2mol L -1 mixed alkaline solution.
[0044] (2) Add 100 mL of deionized water into the reaction kettle as the reaction base liquid, and add ammonia water to adjust the pH to about 11.0. The stirring speed was set to 550 r / min, and the reaction temperature was set to 55°C. Pump the mixed salt solution and mixed alkali solution into the reaction kettle slowly and uniformly, keep the pH stable at 11.0, and the feeding time is 24 hours. After the feeding, continue to pass argon gas and stir for 8 hours. After the reaction is completed, the precipitate is filtered, washed, and dried to obtain a nickel-cobalt cathode material precursor with a molecular formula of Ni 0.92...
Embodiment 2
[0054] (1) Weigh NiSO in the ratio of Ni:Co=92:8 in molar ratio 4 ·6H 2 O and CoSO 4 ·7H 2 O, the total concentration of Ni and Co ions is 4mol L with deionized water -1 mixed salt solution, and then use deionized water to prepare NaOH and ammonia water in a molar ratio of 3:1 to make the NaOH concentration 0.2mol L -1 mixed alkaline solution.
[0055] (2) Add 100 mL of deionized water into the reaction kettle as the reaction base liquid, and add ammonia water to adjust the pH to about 11.0. The stirring speed was set to 600 r / min, and the reaction temperature was set to 55°C. Pump the mixed salt solution and mixed alkali solution into the reaction kettle slowly and uniformly, keep the pH stable at 11.0, and the feeding time is 24 hours. After the feeding, continue to pass argon gas and stir for 8 hours. After the reaction is completed, the precipitate is filtered, washed, and dried to obtain a nickel-cobalt cathode material precursor with a molecular formula of Ni 0.92...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com