Solid beverage containing recombinant human auxin microcapsules and preparation method thereof
A technology for solid beverages and growth hormones, which is used in medical preparations containing active ingredients, microcapsules, and medical preparations without active ingredients, etc., to achieve high drug embedding rate, uniform and controllable particle size, and improved bone strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
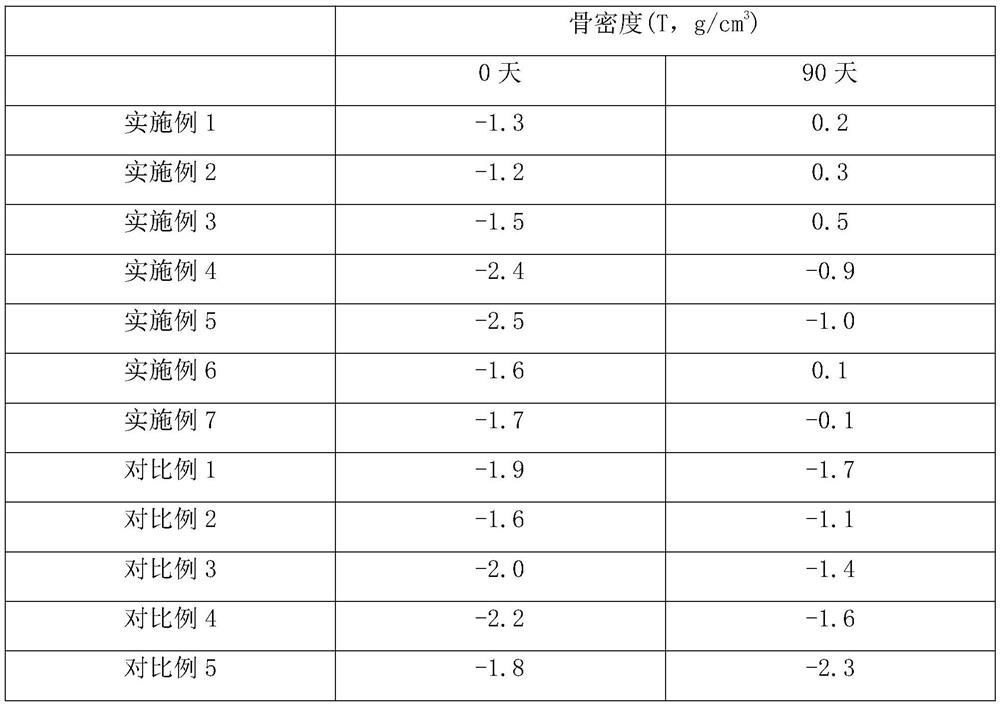
Examples
Embodiment 1
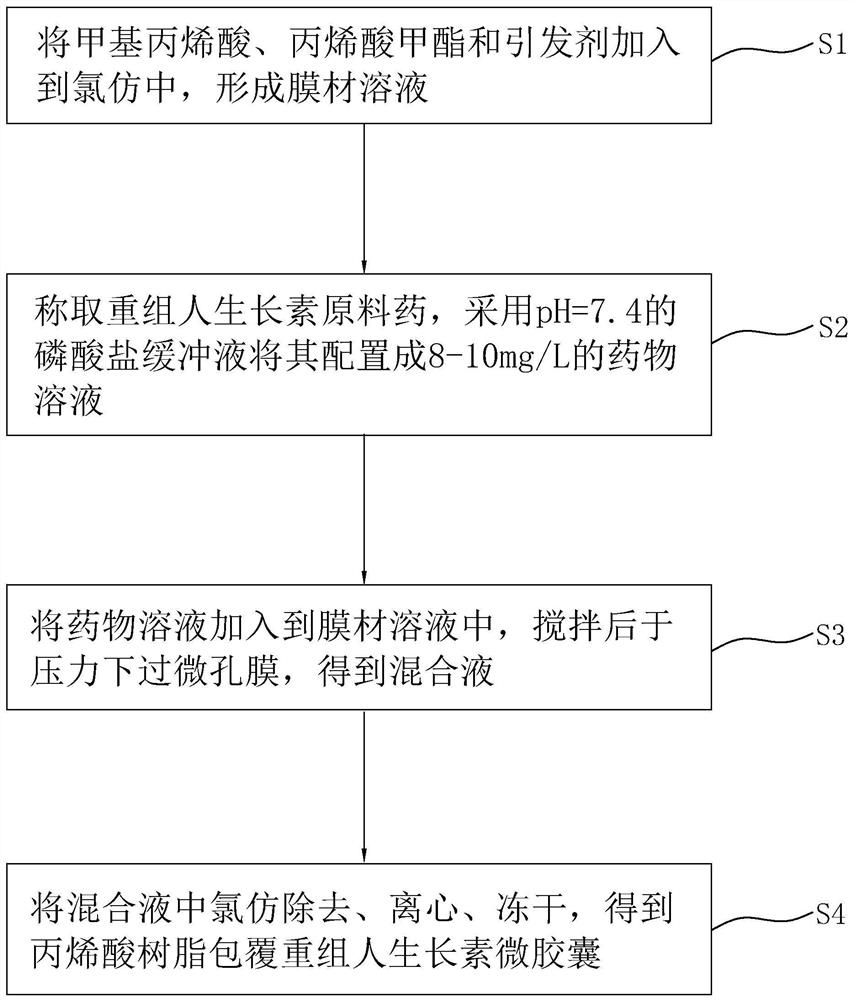
[0049] Example 1: as figure 1 , a solid beverage containing recombinant human growth hormone microcapsules, each component and its corresponding parts by weight are shown in Table 1, and are prepared by the following steps:
[0050] (1) sieving: pass each raw material component through a 20-mesh sieve, for subsequent use;
[0051] (2) Weighing: according to the formula dosage, the raw materials are weighed in turn. During the weighing process, one person weighs the material and one person reviews it;
[0052] (3) premix: vitamin D, sucralose and recombinant human growth hormone microcapsules are mixed in equal amounts for 5min to obtain a premix;
[0053] (4) Mixing: mix the premix with calcium gluconate, casein phosphopeptide, non-dairy creamer, anhydrous citric acid, compound essence, xanthan gum and the remaining vitamin D and sucralose for 30min; wherein the compound essence is composed of The weight ratio is 2:1:1 mixed with yogurt flavor powder, blueberry flavor and po...
Embodiment 2-6
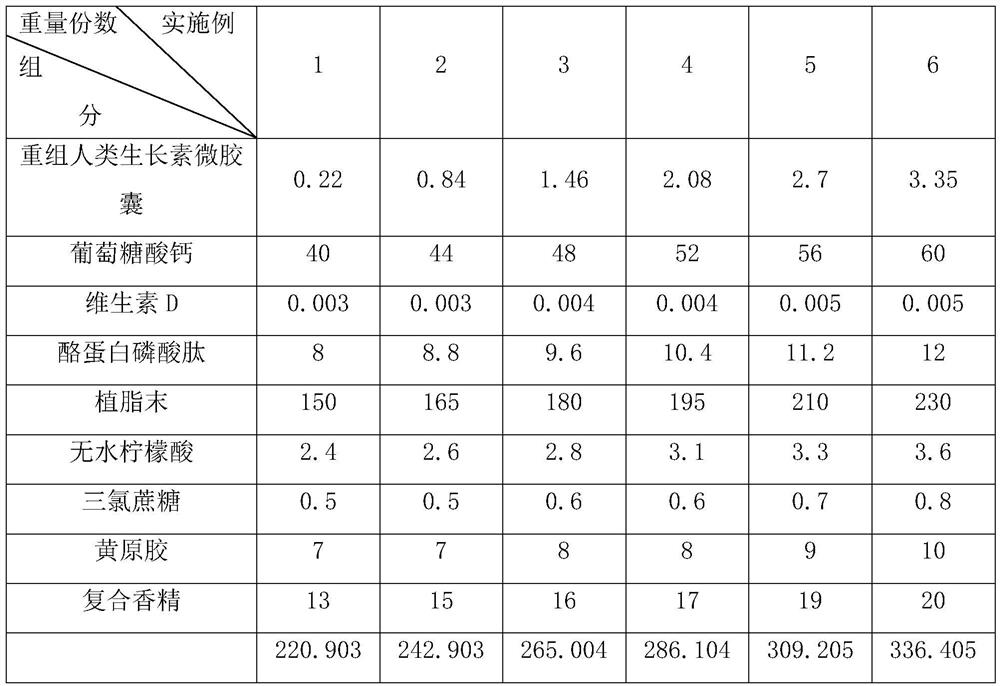
[0061] Example 2-6: A solid beverage containing recombinant human growth hormone microcapsules, which is different from Example 1 in that each component and its corresponding parts by weight are shown in Table 1.
[0062] Table 1 Components and their parts by weight in Examples 1-6
[0063]
Embodiment 7
[0064] Embodiment 7: a solid beverage containing recombinant human growth hormone microcapsules, which is different from embodiment 1 in that the solid beverage is prepared by the following steps:
[0065] (1) sieving: pass each raw material component through a 20-mesh sieve, for subsequent use;
[0066] (2) Weighing: according to the formula dosage, the raw materials are weighed in turn. During the weighing process, one person weighs the material and one person reviews it;
[0067] (3) premix: vitamin D, sucralose and recombinant human growth hormone microcapsules are mixed in equal amounts for 10min to obtain a premix;
[0068] (4) Mixing: mix the premix with calcium gluconate, casein phosphopeptide, non-dairy creamer, anhydrous citric acid, compound essence, xanthan gum and the remaining vitamin D and sucralose for 45min; wherein the compound essence is composed of The weight ratio is 8:1:1 mixed with yogurt flavor powder essence, blueberry essence and powder fresh creamer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com