Method for efficiently infecting photosensory cells of macaque by using ShH10-serotype-gland-related virus
A technology of photoreceptor cells and viruses, which is applied in the fields of cell biology and gene therapy, and can solve the problems of tissue specificity and inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1.ShH10 AAV virus
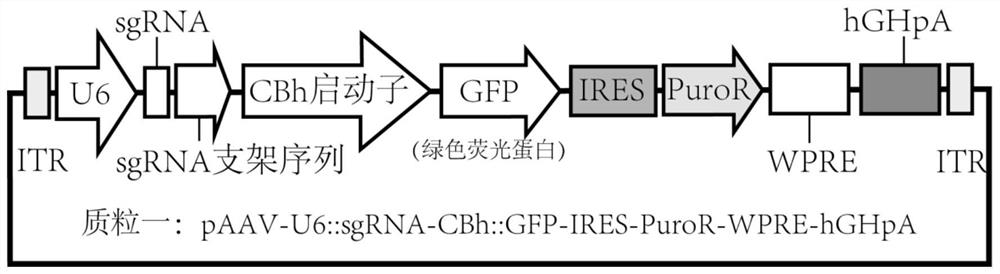
[0045] The pAAV-GFP vector is an adeno-associated virus vector expressing green fluorescent protein. Its purpose is to visualize the cells infected by the virus. It can be any form of adeno-associated virus vector. The vector map is as follows: Figure 1 As shown, the sequence is shown in SEQ ID NO:2. pAAV-GFP vector, ShH10 (SEQ ID NO:3) and pHelper (SEQ ID NO:4) three plasmids were simultaneously transfected (via cationic liposomes) into HEK293T cells to complete AAV virus packaging, 60 hours after transfection The cells were collected for the next step of virus purification. The entire virus preparation method can refer to the literature PMID: 17406430. The cell nuclei containing AAV were sonicated to release virus particles, followed by iodixanol density gradient ultracentrifugation (SW32 rotor, 32000rpm, 4h, 18°C) to purify the virus, and the virus located in 40% density was sucked, and then used Concentrate with A...
Embodiment 2
[0047] Example 2. Efficient infection of rhesus monkey photoreceptor cells with ShH10 AAV virus
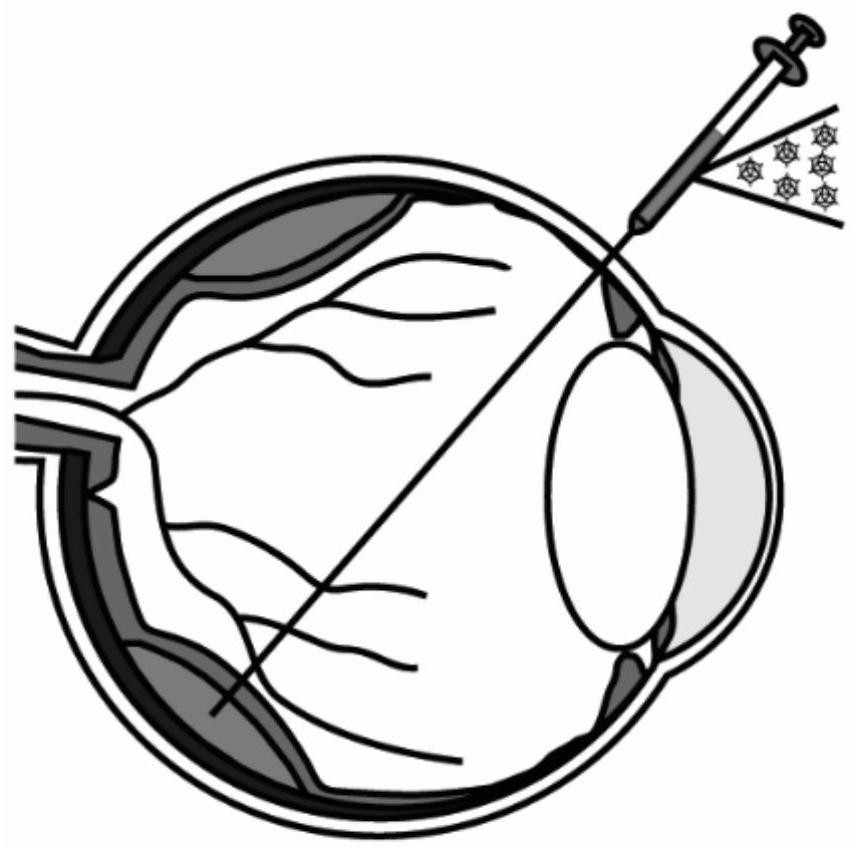
[0048] The virus obtained in Example 1 was injected into the subretinal space of macaques through subretinal injection, such as figure 2 As shown, adult rhesus monkeys were anesthetized by intramuscular injection of ketamine (10 mg / kg) and sodium pentobarbital (40 mg / kg), and propofol (10–12 mg kg -1 h -1 ) for continuous anesthesia. We perforated two holes in the corneal sclera rim to insert a modified Hamilton needle (38G, 40G) and illumination fiber optics, while vitrectomy was performed on macaque eyes to reduce intraocular pressure before AAV virus injection. For a single injection site, the injection volume of AAV virus is 30-50ul, and within three weeks, the injected blisters can be completely absorbed.
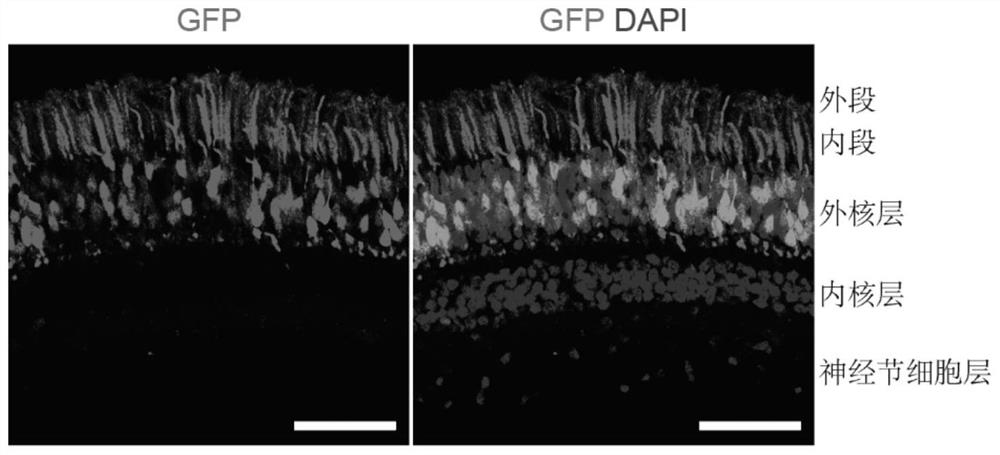
[0049] Three months after the virus injection, macaques were sacrificed for sampling, and the retina was dissected and frozen sectioned for microscopic observation (Le...
Embodiment 3
[0050] Example 3. Specific infection of macaque photoreceptor cells with ShH10 AAV virus
[0051] As in Example 2, ShH10 AAV was packaged and injected into the subretinal space of rhesus monkeys. In this example, mKate2 red fluorescent protein was used as a reporter gene.
[0052] Six months after the virus injection, macaques were sacrificed and sampled. The retina was dissected, frozen and sectioned, and stained for observation. It can be seen that only the photoreceptor cells express red fluorescence, as shown in Figure 4 As shown, no red fluorescent expression was seen in other retinal cell types, especially pigment epithelial cells. The red fluorescence is mKate2 red fluorescent protein expressed by AAV. It can be seen that mKate2 is only expressed in macaque photoreceptor cells, but not in other cell types, especially pigment epithelial cells. This shows that ShH10 AAV is very specific in infecting macaque photoreceptor cells, which improves the safety of genetic manip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com