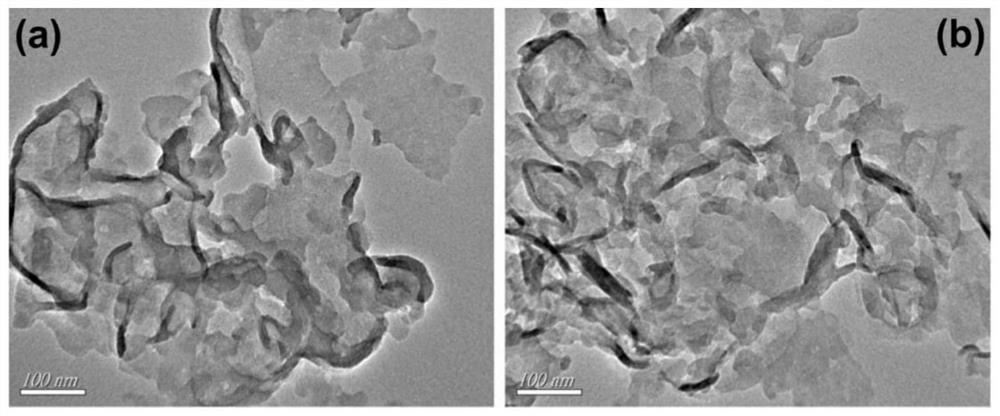
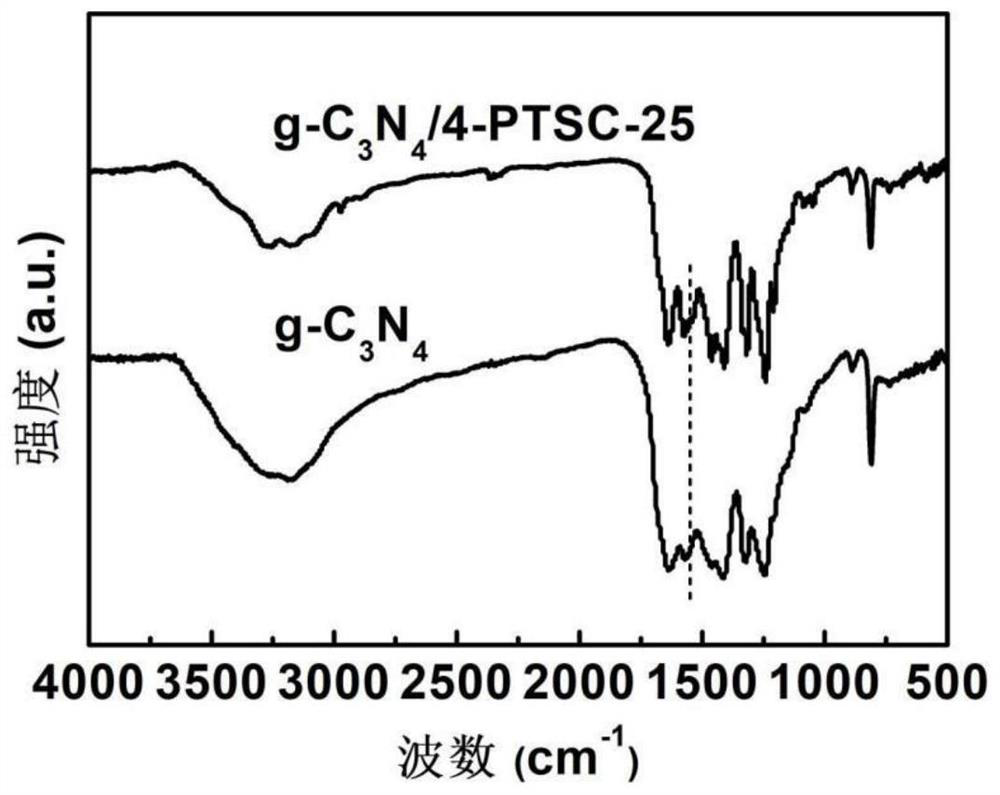
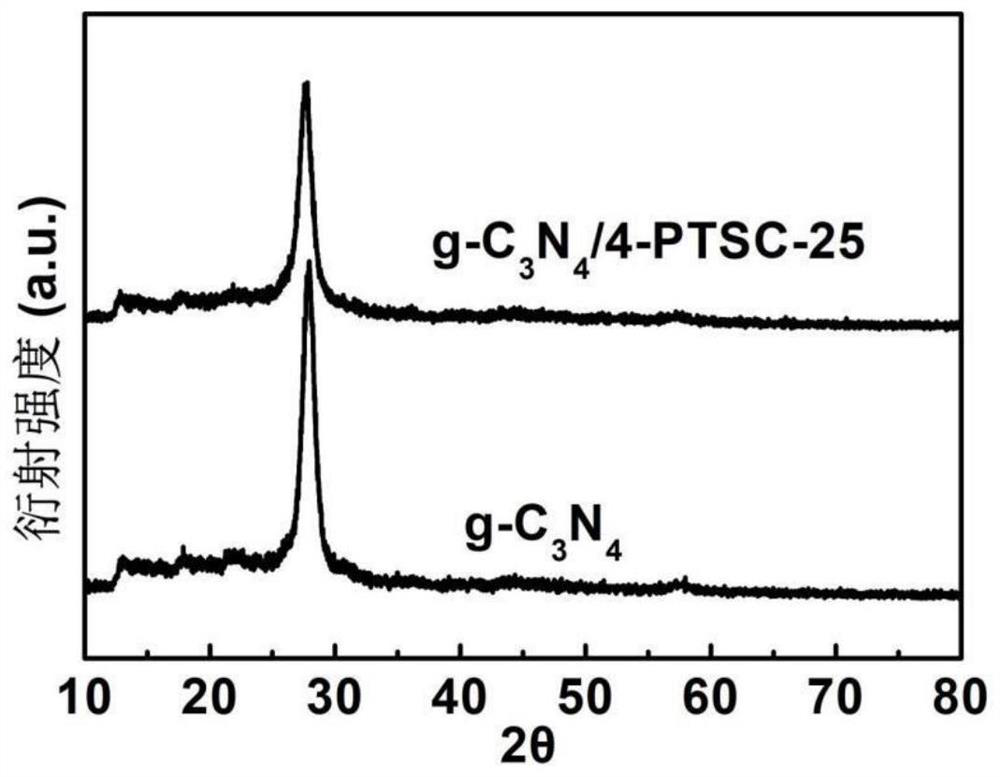
Nonmetallic functional group edge modified composite photocatalyst as well as preparation method and application thereof
A technology of composite light and catalyst, used in non-metallic elements, chemical instruments and methods, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: g-C 3 N 4 Preparation of photocatalyst and its photolysis of water for hydrogen production
[0028] (1) Preparation of g-C 3 N 4 Nanosheets:
[0029] Put a certain amount of urea in an oven at 70°C to dry overnight. The dried urea is ground into powder and put into a crucible with a lid. -1 The heating rate was raised to 550°C and calcined for 4h. After cooling down to room temperature, g-C is obtained 3 N 4 . At the same time, it was tried at 2.5°C min -1 The heating rate was increased to 500 ° C for 4 h and 2.5 ° C min -1 The heating rate was increased to 600 ° C for 2 h to prepare g-C 3 N 4 .
[0030] (2) g-C 3 N 4 Composite photocatalyst photolysis of water for hydrogen production:
[0031] 50mg g-C 3 N 4 Nanosheets were uniformly dispersed in 100ml 20vol% triethanolamine (TEOA) aqueous solution with pH=11.4, and added according to g-C 3 N 4 Add H to 3% of the mass of the nanosheets 2 PtCl 6 ·H 2 O (1.5ml, 1mg / ml Pt) in water. After...
Embodiment 2
[0032] Example 2: g-C 3 N 4 Preparation of / 4-PTSC-5 photocatalyst and its photolysis of water for hydrogen production
[0033] (1) Preparation of g-C 3 N 4 / 4-PTSC-5 photocatalyst:
[0034] Weigh 20g of dried urea and 5mg of 4-PTSC, grind them in a mortar, disperse 4-PTSC evenly in urea, put them into a crucible, and heat them in a muffle furnace from room temperature at 2.5°C min -1 The heating rate was raised to 550°C and calcined for 4h. After cooling down to room temperature, g-C is obtained 3 N 4 / 4-PTSC-5. At the same time, it was tried at 2.5°C min -1 The heating rate was increased to 500 ° C for 4 h and 2.5 ° C min -1 The heating rate was increased to 600 ° C for 2 h to prepare g-C 3 N 4 / 4-PTSC-5.
[0035] (2) g-C 3 N 4 / 4-PTSC-5 composite photocatalyst photolysis of water for hydrogen production:
[0036] 50mg g-C 3 N 4 / 4-PTSC-5 nanosheets are uniformly dispersed in 100ml 20vol% pH=11.4 triethanolamine (TEOA) aqueous solution, and add g-C 3 N 4 ...
Embodiment 3
[0037] Example 3: g-C 3 N 4 Preparation of / 4-PTSC-15 photocatalyst and its photolysis of water for hydrogen production
[0038] (1) Preparation of g-C 3 N 4 / 4-PTSC-15 photocatalyst:
[0039] Weigh 20g of dried urea and 15mg of 4-PTSC, grind them in a mortar, disperse 4-PTSC evenly in urea, put them into a crucible, and heat them in a muffle furnace from room temperature at 2.5°C min -1 The heating rate was raised to 550°C and calcined for 4h. After cooling down to room temperature, g-C is obtained 3 N 4 / 4-PTSC-15. At the same time, it was tried at 2.5°C min -1 The heating rate was increased to 500 ° C for 4 h and 2.5 ° C min -1 The heating rate was increased to 600 ° C for 2 h to prepare g-C 3 N 4 / 4-PTSC-15.
[0040] (2) g-C 3 N 4 / 4-PTSC-15 composite photocatalyst photolysis of water for hydrogen production:
[0041] 50mg g-C 3 N 4 / 4-PTSC-15 nanosheets are uniformly dispersed in 100ml 20vol% pH=11.4 triethanolamine (TEOA) aqueous solution, and add g-C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com