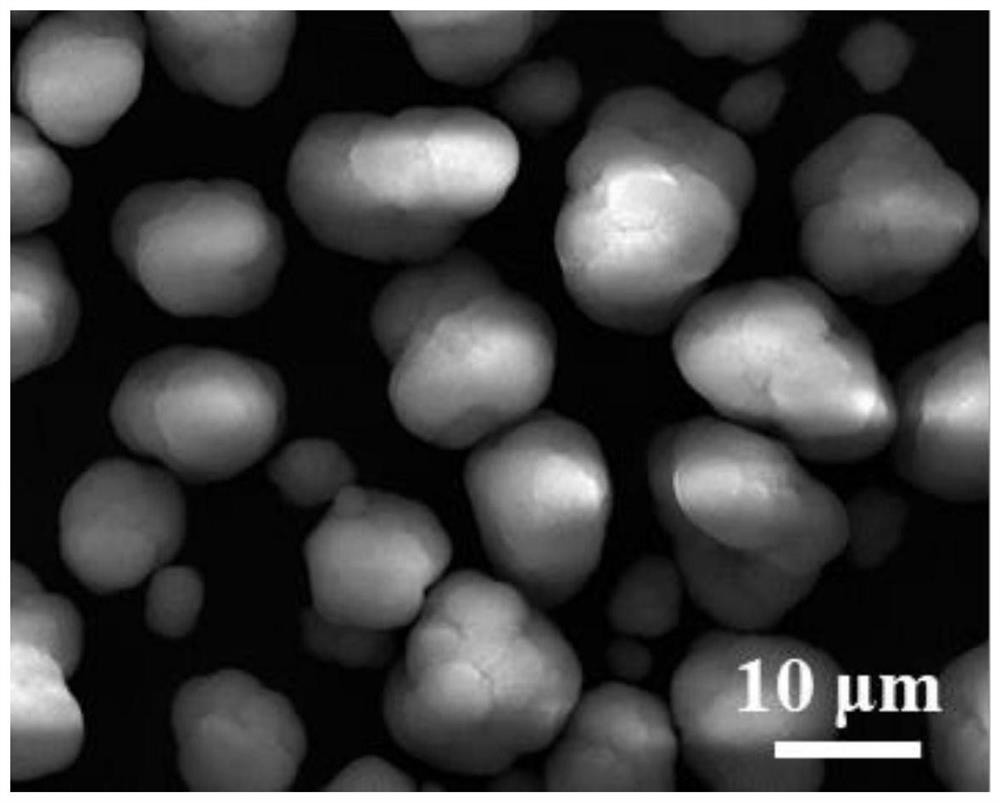
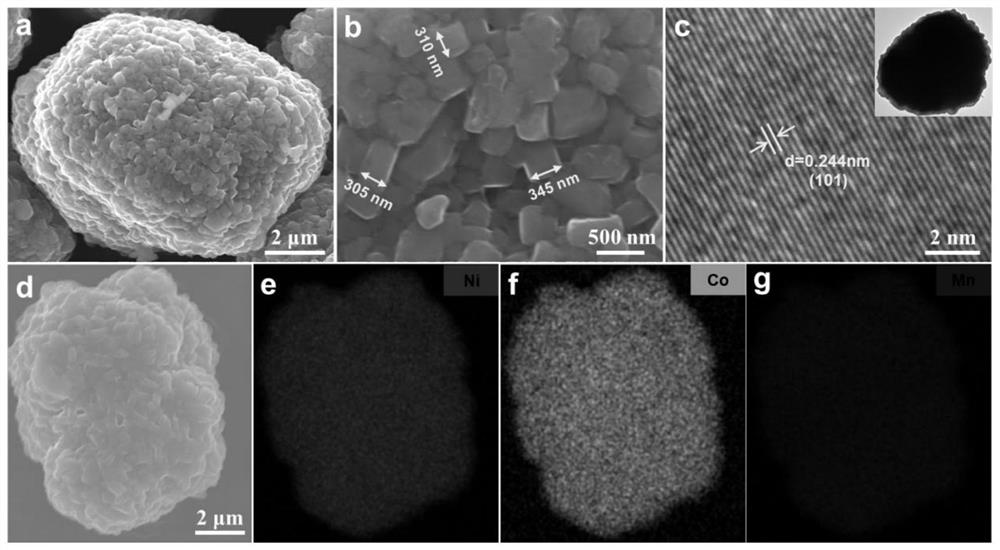
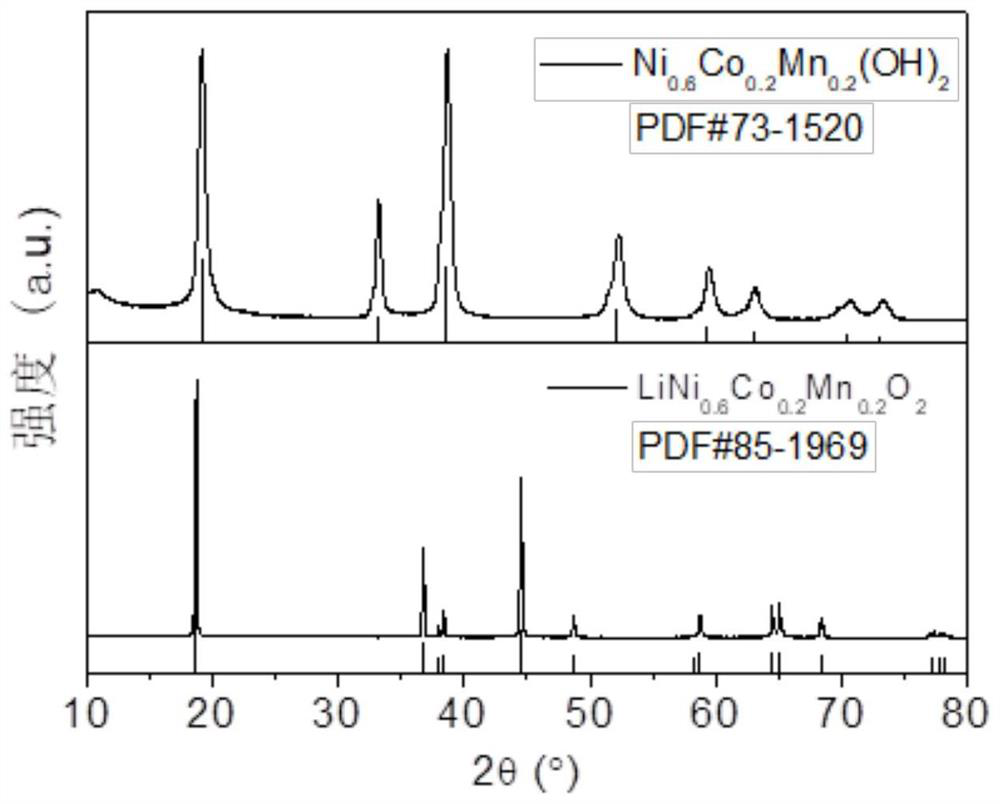
Method for preparing ternary positive electrode material of high-performance lithium ion battery at low ammonia concentration
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, active material electrodes, positive electrodes, etc., can solve problems such as low tap density, environmental pollution, and health hazards for workers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Take NiSO with a molar ratio of 0.6:0.2:0.2 4 ·6H 2 O. COSO 4 ·7H 2 O, MnSO 4 ·H 2 O dissolved in deionized water to prepare 1.25mol L -1 Mixed salt solution of nickel, cobalt and manganese.
[0041] 2) Add ammonium nitrate (concentration in the mixed solution is 0.125mol L to the above step 1) in the salt solution -1 ) and stirring and dissolving to obtain a uniformly mixed salt and complexing agent mixed solution.
[0042] 3) Adjust the pH value of the mixed solution in the above step 2) to 1.0 by adding nitric acid.
[0043] 4) Mix the solution and 8mol L of the above step 3) through a peristaltic pump -1 The NaOH solution was continuously pumped into 0.8L ammonia concentration of 0.1mol L -1 In the 2L continuous co-precipitation reactor of the bottom liquid ammonia water, adjust the mixed solution feed rate in step 3) to be 5.76mL min -1 , the feeding rate of NaOH solution is 1.44mL min -1 , so as to control the total ammonia concentration in the react...
Embodiment 2
[0050] The specific steps and reaction conditions are the same as in Example 1, except that the reaction pH is controlled to be 10.0 in step 4).
Embodiment 3
[0052]The specific steps and reaction conditions are the same as in Example 1, except that the reaction pH is controlled to be 10.5 in step 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Coulombic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com