Preparation process of 1, 1-dichloro-3, 3, 3-trifluoropropene
A preparation process, a technology of trifluoropropene, which is applied in the preparation of halogenated hydrocarbons, catalyst activation/preparation, organic chemistry, etc., and can solve the problems of low conversion rate of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: (catalytic system: 15wt%Ru / 85%Cr 2 o 3 ;3wt%Sm 2 o 3 , 2wt% SmCl 3 , 4wt% ZnO, wherein the mass of the main catalyst is 100%)
[0051] The main catalyst is prepared by impregnation method: the nitrate of the active component Ru is stirred and dissolved in deionized water to obtain a metal salt solution, that is, the impregnation solution; the dried multi-level micro / mesoporous Cr 2 o 3 The carrier was impregnated in the impregnating solution for 4 hours at room temperature, then filtered, and dried in a drying oven at 110°C for 9 hours to obtain a precursor. Finally, the obtained precursor was calcined under an inert gas argon atmosphere, and calcined at 500°C for 5 hours to obtain the main catalyst. Among them, the multi-level micro / mesoporous Cr 2 o 3 The specific surface area of the carrier is 65.5m 2 / g, the average pore diameter is 8.5nm, 65.5% of the pore diameter is 0.2-2nm, and 12.7% of the pore diameter is 15-50nm.
[0052] By physicall...
Embodiment 2
[0054] Embodiment 2: (catalytic system: 15wt%Pd / 85%Cr 2 o 3 ;3wt% La 2 o 3 , 2wt% LaCl 3 , 4wt% ZnO, wherein the mass of the main catalyst is 100%)
[0055] The main catalyst is prepared by impregnation method: the nitrate of the active component Pd is stirred and dissolved in deionized water to obtain a metal salt solution, that is, the impregnation solution; the dried multi-level micro / mesoporous Cr 2 o 3 The carrier was impregnated in the impregnating solution for 4 hours at room temperature, then filtered, and dried in a drying oven at 110°C for 9 hours to obtain a precursor. Finally, the obtained precursor was calcined under an inert gas argon atmosphere, and calcined at 500°C for 5 hours to obtain the main catalyst. Among them, the multi-level micro / mesoporous Cr 2 o 3 The specific surface area of the carrier is 55.8m 2 / g, the average pore diameter is 9.5nm, 55.5% of the pore diameter is 0.2-2nm, and 15.2% of the pore diameter is 15-50nm.
[0056] By physical...
Embodiment 3
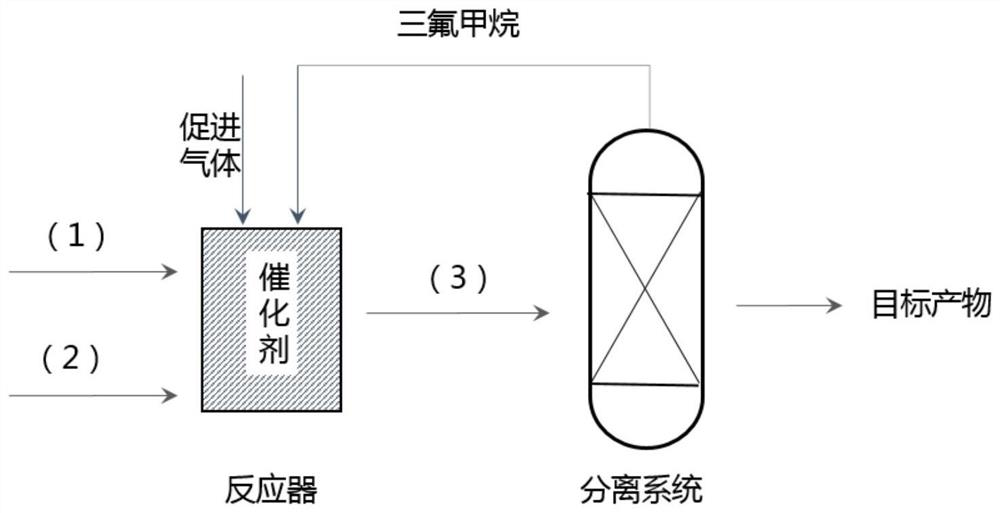
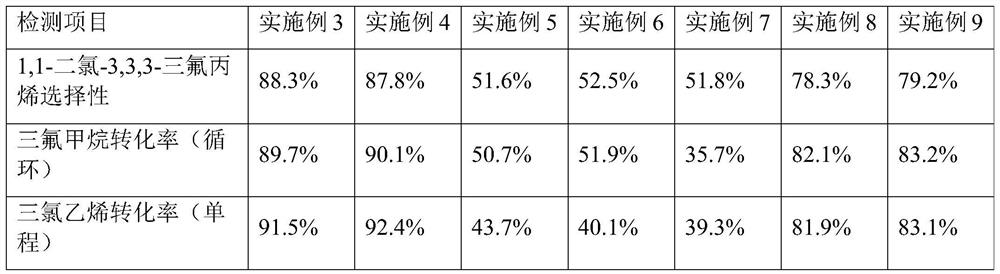
[0075] Trifluoromethane raw material stream (1) and trichloroethylene raw material stream (2) are passed into the reactor respectively, and in the presence of the catalytic system of Example 1, gas phase reaction generates reaction product stream (3). The heating temperature of the reactor is 400°C, the raw material trifluoromethane is excessive, and the molar ratio of trifluoromethane and trichloroethylene is 1.5:1. In the reaction stage, a reaction-promoting gas is added, and the reaction-promoting gas is Cl with a molar ratio of 20%. 2 and 80% molar CO 2 , the molar ratio of reaction-promoting gas to trifluoromethane is 0.01:1. The reaction product stream (3) is sent to a separation system for separation to obtain the target product 1,1-dichloro-3,3,3-trifluoropropene.
[0076] Wherein, excess trifluoromethane passes through an extraction tower, is purified and circulated into the initial reactor as a reaction raw material. The extract liquid in the extraction tower is i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com