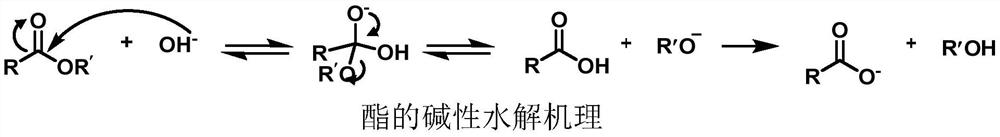
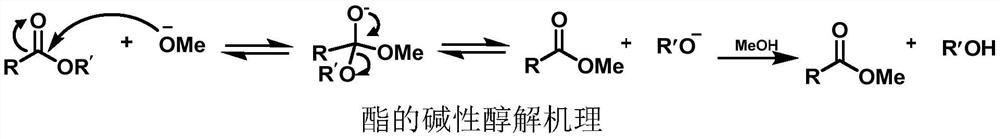
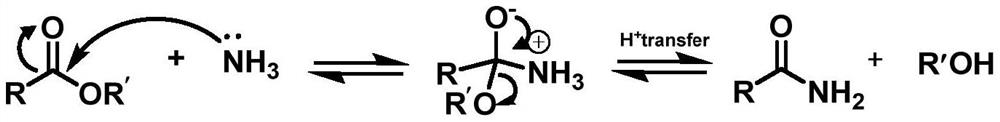
Deacylation method of sucralose-6-acetate
A technology of sucralose and acetate, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of impurity removal not being well mentioned, product destruction, sugar substance destruction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Using existing technology to prepare sucralose-6-acetate from sucrose-6-acetate
[0031] Dissolve 6.8kg of phosgene in 11.3kg of n-octane to obtain phosgene n-octane solution, and slowly add it dropwise to the N,N-dimethyl In the formamide (DMF, 24.9kg) solution, the dropwise addition is completed, and the reaction mixture is stirred at room temperature until the system becomes a clear homogeneous solution; then the liquid-liquid two-phase mixture is heated and reacted at 96°C for 13 hours, the reaction is stopped, and the system is cooled To room temperature, stand for stratification, wherein the upper layer is an inert non-polar solvent layer, and the lower layer is a DMF solution layer containing the product; the lower layer is neutralized with ammonia gas or sodium ethoxide ethanol solution, filtered to obtain a filtrate, and then the filtrate is concentrated to obtain a concentrated 5.2kg (contains 2.34kg of sucralose-6-acetate after testing).
Embodiment 2
[0032] Embodiment 2, Lewis acid (anhydrous ZnCl 2 )-mediated deacylation and post-processing
[0033] Take 2.6kg of the neutralized concentrate (containing 1.17kg of sucralose-6-acetate) for deacylation, add 5.2kg of methanol to form a sucralose-6-acetate solution with a content of about 15% , adding anhydrous zinc chloride (0.75kg) and stirring the reaction at an internal temperature of 50° C., the reaction was monitored by HPLC, and the reaction was complete in about 200 minutes.
[0034] Remove methanol from the above solution under reduced pressure, then add 5.2kg of water to dissolve the concentrate, add a small amount of lye (sodium hydroxide) to adjust the pH value to 7.0, so that the zinc salt inside is completely settled in the form of colloid or precipitate, and let stand Filter after 4h, extract the filtrate with 3.5 times EA (ethyl acetate), concentrate, and crystallize through ethyl acetate once (using continuous feeding, pressure 0.07-0.08MPa, internal temperatu...
Embodiment 3
[0035] Embodiment 3, Lewis acid (anhydrous AlCl3 )-mediated deacylation and post-processing
[0036] Take 2.6kg of the neutralized concentrate (containing 1.17kg of sucralose-6-acetate) for deacylation, add 5.2kg of methanol to form a sucralose-6-acetate solution with a content of about 15% , adding anhydrous aluminum chloride (0.75kg) and stirring the reaction at an internal temperature of 20° C., the reaction was monitored by HPLC, and the reaction was complete in about 120 minutes.
[0037] Remove methanol from the above solution under reduced pressure, then add 5.2kg of water to dissolve the concentrate, add a small amount of lye to adjust the pH value to 7.0, so that the aluminum salt inside is completely settled in the form of colloid or precipitation, and after standing for 4 hours, filter to obtain After the filtrate was extracted with 3.5 times of EA, concentrated and crystallized, 1.04 kg of sucralose was obtained with an HPLC purity of 99.9%. The deacylation yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com