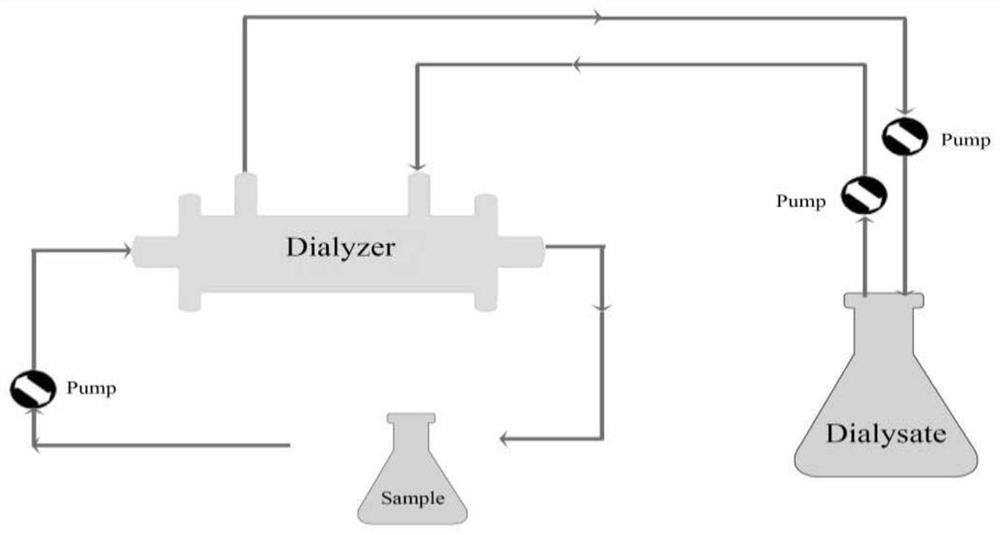
Liposome, dispersion liquid containing liposome, and preparation methods and application of liposome and dispersion liquid
A liposome and dispersion technology, applied in the medical field, can solve problems such as the source of human serum albumin and the limitation of treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method provided by the present invention may include: A) dispersing each raw material component in a solvent to provide a premix. The solvent used in the preparation method can generally be a good solvent for the raw material components, usually an organic solvent, specifically a halogenated alkane solvent, an alcohol solvent, etc. In a preferred embodiment of the present invention, the solvent It can be a combination of one or more of dichloromethane, chloroform, methanol or ethanol, etc., and the amount of solvent used can be 25-75mL / 1g solid, 25-35mL / 1g solid, 35-35mL / 1g solid, etc. 45mL / 1g solid, 45-55mL / 1g solid, 55-65mL / 1g solid, or 65-75mL / 1g solid.
[0042] In the preparation method provided by the present invention, it may also include: B) removing the solvent in the premix provided in step A), so as to provide a phospholipid film. The method for removing the solvent should be known to those skilled in the art, for example, the solvent in the p...
Embodiment 1
[0052] Preparation of liposomes:
[0053] Soybean lecithin liposomes and linoleic acid-modified liposomes were prepared by film hydration method.
[0054] In the liposomes modified with linoleic acid, soybean lecithin (analytical pure, Shanghai Aiweite Pharmaceutical Technology Co., Ltd.), cholesterol (analytical pure, Shanghai Yuanju Biotechnology Co., Ltd.), Tween-80 (analytical pure, Shanghai Yuanju Biotechnology Co., Ltd.) The ratio between Yuanju Biotechnology Co., Ltd.) and linoleic acid (analytical grade, Sigma) is 9:3:4:3, in soybean lecithin liposomes (that is, liposomes without linoleic acid modification) , the ratio between soybean lecithin, cholesterol, and Tween-80 is 12:5:3, and each raw material component is dissolved in dichloromethane, and vacuum evaporated overnight. Then, the obtained dry film was hydrated with PBS buffer solution (addition amount: 25 mL / 1 g solid), and homogenized with a high-pressure homogenizer, with a homogenization pressure of 60 bar a...
Embodiment 2
[0056] Characterization of linoleic acid liposomes:
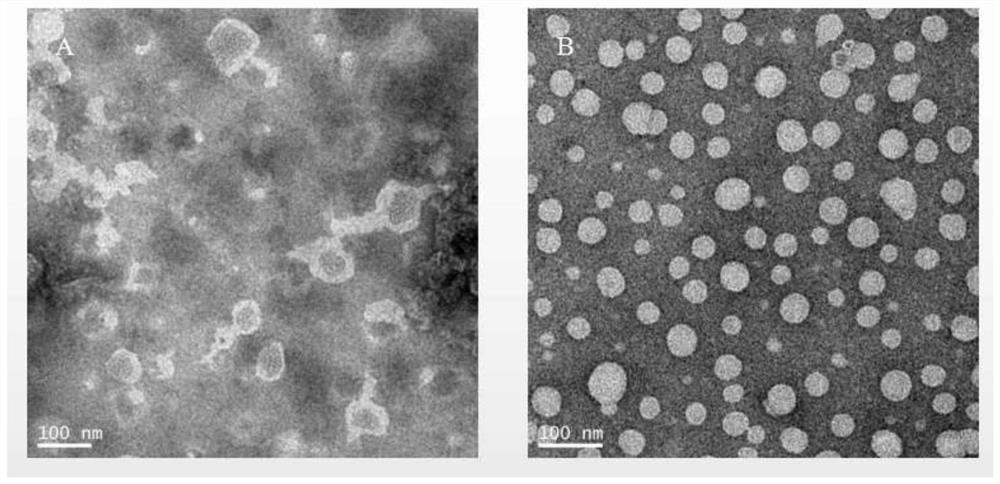
[0057] Stain with phosphotungstic acid, and observe the morphology of linoleic acid liposomes under a transmission electron microscope. The particle size and potential of liposomes were measured using a Zetasizer instrument.
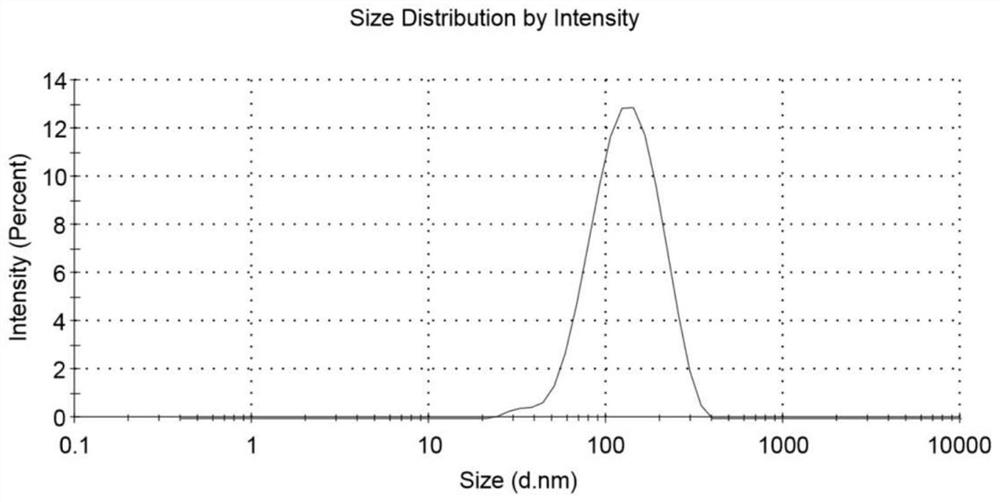
[0058] The diameter range of most liposomes is 100-200nm, the distribution range is about 109.1±60.28nm, the average particle size of linoleic acid liposome is about 116.4±0.99nm, and the dispersion index (PDI) is 0.15±0.01( figure 2 ). The structure of phospholipid bilayer wrapped water-based inner core can be seen under transmission electron microscope ( image 3 ). The liposome solution was stable at room temperature for 14 days without precipitation, and the stability of the liposome was partly due to its negative surface potential (-6.96 ± 0.68mV) ( Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com