Preparation method of capmatinib
A compound and methylation technology, applied in the field of drug synthesis, can solve the problems of high energy consumption, high equipment corrosion, high environmental protection pressure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
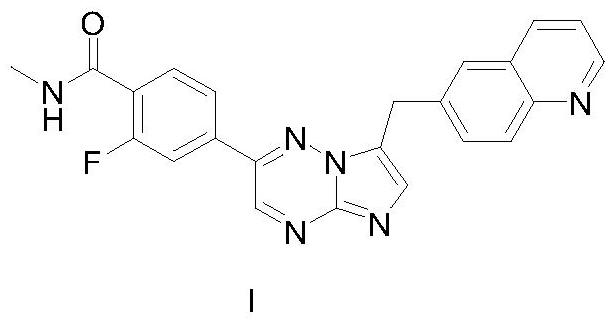
[0034] The preparation of embodiment 1 capmatinib
[0035]
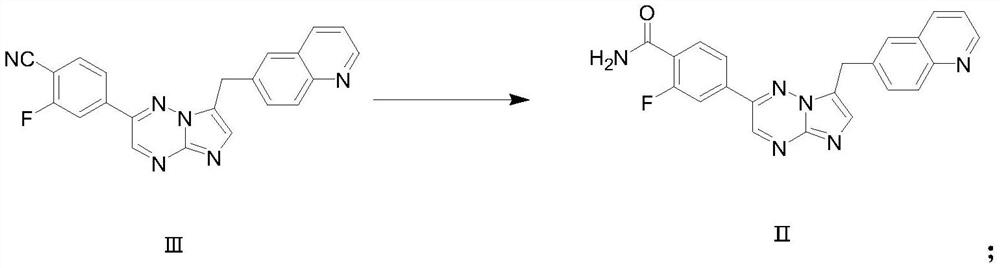
[0036] Step 1: Formula II Compound preparation
[0037] Add 38.2g (0.1mol) formula III compound to 2L reaction bottle, methylene chloride 200ml and water 100ml, 'tetrabutylammonium chloride 2g, 30% hydrogen peroxide 150ml, 20% sodium hydroxide aqueous solution 200ml, room temperature reaction 5-6 After 1 hour, add 100ml of dichloromethane after the completion of the reaction, stir and separate the liquids, wash the dichloromethane phase once with aqueous sodium sulfite solution, wash once with clear water, and concentrate the dichloromethane phase to dryness to obtain 35.1g of the title compound. The yield is 88%, and the HPLC purity is 98.5%. . MS (ESI): [M+1] = 399.5.
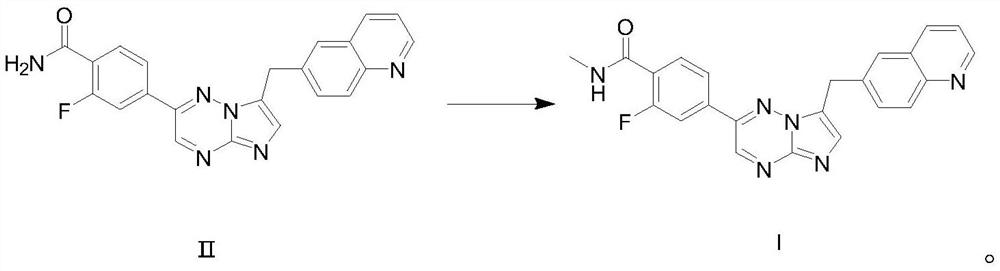
[0038] Step 2: Preparation of capmatinib
[0039] Add 500ml of DMF and 20.0g (0.05mol) of the compound of formula II to a 2L reaction flask, stir to dissolve, cool to 0-5°C, add 2.4g (0.06mol) of 60% sodium hydride in mineral oil in batches...
Embodiment 19
[0041] First, 2-fluoro-N-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-B]-[1,2, 4] Triazin-2-yl] benzoic acid, the specific method is as follows:
[0042]Add 2500ml of concentrated hydrochloric acid, 100ml of water, and 277.5g (0.73mol) of the compound of formula III to a 5000ml reaction flask, stir and dissolve, heat up to 100°C and reflux for 18 hours, LC-MS tracks the end of the reaction, cools the reaction solution to 80°C, and transfers to 10L stirring equipment, add 2500ml of water, cool down and crystallize, after the solid precipitates, continue to cool to 0°C, filter to obtain the solid, wash the filter cake with 1N hydrochloric acid, and dry the solid in vacuum to obtain 262.3g of the title compound, with a yield of 90%. MS (ESI): [M+1] = 400.1.
[0043] Then, prepare capmatinib with reference to Example 20 of US2009291956A1, the specific method is as follows:
[0044] In a 10L reaction vessel, add DMF3700ml, add 431.4g (0.914mol) 2-fluoro-N-methyl-4-[7-[(quinolin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com